Saturation Mutagenesis and Molecular Modeling: The Impact of Methionine 182 Substitutions on the Stability of β-Lactamase TEM-1
- PMID: 39062934
- PMCID: PMC11276661
- DOI: 10.3390/ijms25147691
Saturation Mutagenesis and Molecular Modeling: The Impact of Methionine 182 Substitutions on the Stability of β-Lactamase TEM-1
Abstract
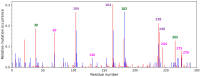
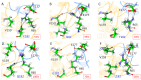
Serine β-lactamase TEM-1 is the first β-lactamase discovered and is still common in Gram-negative pathogens resistant to β-lactam antibiotics. It hydrolyzes penicillins and cephalosporins of early generations. Some of the emerging TEM-1 variants with one or several amino acid substitutions have even broader substrate specificity and resistance to known covalent inhibitors. Key amino acid substitutions affect catalytic properties of the enzyme, and secondary mutations accompany them. The occurrence of the secondary mutation M182T, called a "global suppressor", has almost doubled over the last decade. Therefore, we performed saturating mutagenesis at position 182 of TEM-1 to determine the influence of this single amino acid substitution on the catalytic properties, thermal stability, and ability for thermoreactivation. Steady-state parameters for penicillin, cephalothin, and ceftazidime are similar for all TEM-1 M182X variants, whereas melting temperature and ability to reactivate after incubation at a higher temperature vary significantly. The effects are multidirectional and depend on the particular amino acid at position 182. The M182E variant of β-lactamase TEM-1 demonstrates the highest residual enzymatic activity, which is 1.5 times higher than for the wild-type enzyme. The 3D structure of the side chain of residue 182 is of particular importance as observed from the comparison of the M182I and M182L variants of TEM-1. Both of these amino acid residues have hydrophobic side chains of similar size, but their residual activity differs by three-fold. Molecular dynamic simulations add a mechanistic explanation for this phenomenon. The important structural element is the V159-R65-E177 triad that exists due to both electrostatic and hydrophobic interactions. Amino acid substitutions that disturb this triad lead to a decrease in the ability of the β-lactamase to be reactivated.
Keywords: M182X mutation; antibiotic resistance; molecular modeling; saturating mutagenesis; thermostability; β-lactamase TEM-1.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


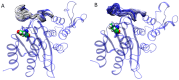
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

