Torque Teno Virus: A Promising Biomarker in Kidney Transplant Recipients
- PMID: 39062987
- PMCID: PMC11277443
- DOI: 10.3390/ijms25147744
Torque Teno Virus: A Promising Biomarker in Kidney Transplant Recipients
Abstract
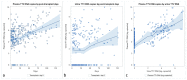
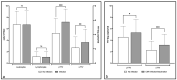
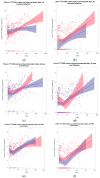
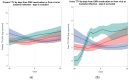
Torque Teno Virus (TTV) is a ubiquitous component of the human virome, not associated with any disease. As its load increases when the immune system is compromised, such as in kidney transplant (KT) recipients, TTV load monitoring has been proposed as a method to assess immunosuppression. In this prospective study, TTV load was measured in plasma and urine samples from 42 KT recipients, immediately before KT and in the first 150 days after it. Data obtained suggest that TTV could be a relevant marker for evaluating immune status and could be used as a guide to predict the onset of infectious complications in the follow-up of KT recipients. Since we observed no differences considering distance from transplantation, while we found a changing trend in days before viral infections, we suggest to consider changes over time in the same subjects, irrespective of time distance from transplantation.
Keywords: Torque Teno Virus; anellovirus; immunosuppression; immunosuppressive therapy; infection; kidney transplantation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

