Mycobacterial Biofilm: Mechanisms, Clinical Problems, and Treatments
- PMID: 39063012
- PMCID: PMC11277187
- DOI: 10.3390/ijms25147771
Mycobacterial Biofilm: Mechanisms, Clinical Problems, and Treatments
Abstract
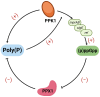
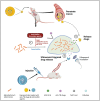
Tuberculosis (TB) remains a threat to human health worldwide. Mycobacterium tuberculosis (Mtb) and other nontuberculous mycobacteria (NTM) can form biofilms, and in vitro and animal experiments have shown that biofilms cause serious drug resistance and mycobacterial persistence. Deeper investigations into the mechanisms of mycobacterial biofilm formation and, consequently, the exploration of appropriate antibiofilm treatments to improve the efficiency of current anti-TB drugs will be useful for curing TB. In this review, the genes and molecules that have been recently reported to be involved in mycobacterial biofilm development, such as ABC transporter, Pks1, PpiB, GroEL1, MprB, (p)ppGpp, poly(P), and c-di-GMP, are summarized. Biofilm-induced clinical problems, including biofilm-related infections and enhanced virulence, as well as their possible mechanisms, are also discussed in detail. Moreover, we also illustrate newly synthesized anti-TB agents that target mycobacterial biofilm, as well as some assistant methods with high efficiency in reducing biofilms in hosts, such as the use of nanoparticles.
Keywords: Mycobacterium tuberculosis; antibiofilm; biofilm; drug resistance; mycobacterial biofilm.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Ojha A.K., Baughn A.D., Sambandan D., Hsu T., Trivelli X., Guerardel Y., Alahari A., Kremer L., Jacobs W.R., Jr., Hatfull G.F. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol. Microbiol. 2008;69:164–174. doi: 10.1111/j.1365-2958.2008.06274.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

