The Role of PTEN in Chemoresistance Mediated by the HIF-1α/YY1 Axis in Pediatric Acute Lymphoblastic Leukemia
- PMID: 39063014
- PMCID: PMC11276810
- DOI: 10.3390/ijms25147767
The Role of PTEN in Chemoresistance Mediated by the HIF-1α/YY1 Axis in Pediatric Acute Lymphoblastic Leukemia
Abstract
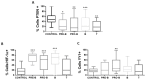
Acute lymphoblastic leukemia (ALL) is the most common childhood cancer. Current chemotherapy treatment regimens have improved survival rates to approximately 80%; however, resistance development remains the primary cause of treatment failure, affecting around 20% of cases. Some studies indicate that loss of the phosphatase and tensin homolog (PTEN) leads to deregulation of the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway, increasing the expression of proteins involved in chemoresistance. PTEN loss results in deregulation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and induces hypoxia-inducible factor 1-alpha (HIF-1α) expression in various cancers. Additionally, it triggers upregulation of the Yin Yang 1 (YY1) transcription factor, leading to chemoresistance mediated by glycoprotein p-170 (Gp-170). The aim of this study was to investigate the role of the PTEN/NF-κB axis in YY1 regulation via HIF-1α and its involvement in ALL. A PTEN inhibitor was administered in RS4;11 cells, followed by the evaluation of PTEN, NF-κB, HIF-1α, YY1, and Gp-170 expression, along with chemoresistance assessment. PTEN, HIF-1α, and YY1 expression levels were assessed in the peripheral blood mononuclear cells (PBMC) from pediatric ALL patients. The results reveal that the inhibition of PTEN activity significantly increases the expression of pAkt and NF-κB, which is consistent with the increase in the expression of HIF-1α and YY1 in RS4;11 cells. In turn, this inhibition increases the expression of the glycoprotein Gp-170, affecting doxorubicin accumulation in the cells treated with the inhibitor. Samples from pediatric ALL patients exhibit PTEN expression and higher HIF-1α and YY1 expression compared to controls. PTEN/Akt/NF-κB axis plays a critical role in the regulation of YY1 through HIF-1α, and this mechanism contributes to Gp-170-mediated chemoresistance in pediatric ALL.
Keywords: Gp-170; HIF-1α; PTEN; YY1; leukemia.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




Similar articles
-
Transcriptional Regulation of Yin-Yang 1 Expression through the Hypoxia Inducible Factor-1 in Pediatric Acute Lymphoblastic Leukemia.Int J Mol Sci. 2022 Feb 2;23(3):1728. doi: 10.3390/ijms23031728. Int J Mol Sci. 2022. PMID: 35163649 Free PMC article.
-
Yin-Yang-1 decreases Fas-induced apoptosis in acute lymphoblastic leukemia under hypoxic conditions: its implications in immune evasion.Bol Med Hosp Infant Mex. 2020;77(4):186-194. doi: 10.24875/BMHIM.19000187. Bol Med Hosp Infant Mex. 2020. PMID: 32713953 English.
-
Regulation of HIF-1α signaling and chemoresistance in acute lymphocytic leukemia under hypoxic conditions of the bone marrow microenvironment.Cancer Biol Ther. 2012 Aug;13(10):858-70. doi: 10.4161/cbt.20838. Epub 2012 Aug 1. Cancer Biol Ther. 2012. PMID: 22785211 Free PMC article.
-
New insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling axis: targeted therapy of γ-secretase inhibitor resistant T-cell acute lymphoblastic leukemia.Cell Signal. 2014 Jan;26(1):149-61. doi: 10.1016/j.cellsig.2013.09.021. Epub 2013 Oct 16. Cell Signal. 2014. PMID: 24140475 Review.
-
The novel role of Yin Yang 1 in the regulation of epithelial to mesenchymal transition in cancer via the dysregulated NF-κB/Snail/YY1/RKIP/PTEN Circuitry.Crit Rev Oncog. 2011;16(3-4):211-26. doi: 10.1615/critrevoncog.v16.i3-4.50. Crit Rev Oncog. 2011. PMID: 22248055 Review.
Cited by
-
YY1/HIF-1α/mROS positive-feedback loop exacerbates glomerular mesangial cell proliferation in mouse early diabetic kidney disease.Acta Pharmacol Sin. 2025 Jul;46(7):1974-1989. doi: 10.1038/s41401-025-01498-7. Epub 2025 Mar 4. Acta Pharmacol Sin. 2025. PMID: 40038466
-
Hyaluronan-Mediated Motility Receptor (HMMR) Overexpression Is Correlated with Poor Survival in Patients with B-ALL.Int J Mol Sci. 2025 Jan 16;26(2):744. doi: 10.3390/ijms26020744. Int J Mol Sci. 2025. PMID: 39859458 Free PMC article.
References
-
- Badowska-Kozakiewicz A.M., Sobol M., Patera J. Expression of multidrug resistance protein P-glycoprotein in correlation with markers of hypoxia (HIF-1α, EPO, EPO-R) in invasive breast cancer with metastasis to lymph nodes. Arch. Med. Sci. 2017;6:1303–1314. doi: 10.5114/aoms.2016.62723. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

