De Novo DNM1L Mutation in a Patient with Encephalopathy, Cardiomyopathy and Fatal Non-Epileptic Paroxysmal Refractory Vomiting
- PMID: 39063023
- PMCID: PMC11277250
- DOI: 10.3390/ijms25147782
De Novo DNM1L Mutation in a Patient with Encephalopathy, Cardiomyopathy and Fatal Non-Epileptic Paroxysmal Refractory Vomiting
Abstract
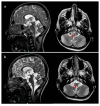
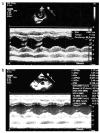
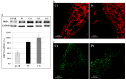
Mitochondrial fission and fusion are vital dynamic processes for mitochondrial quality control and for the maintenance of cellular respiration; they also play an important role in the formation and maintenance of cells with high energy demand including cardiomyocytes and neurons. The DNM1L (dynamin-1 like) gene encodes for the DRP1 protein, an evolutionary conserved member of the dynamin family that is responsible for the fission of mitochondria; it is ubiquitous but highly expressed in the developing neonatal heart. De novo heterozygous pathogenic variants in the DNM1L gene have been previously reported to be associated with neonatal or infantile-onset encephalopathy characterized by hypotonia, developmental delay and refractory epilepsy. However, cardiac involvement has been previously reported only in one case. Next-Generation Sequencing (NGS) was used to genetically assess a baby girl characterized by developmental delay with spastic-dystonic, tetraparesis and hypertrophic cardiomyopathy of the left ventricle. Histochemical analysis and spectrophotometric determination of electron transport chain were performed to characterize the muscle biopsy; moreover, the morphology of mitochondria and peroxisomes was evaluated in cultured fibroblasts as well. Herein, we expand the phenotype of DNM1L-related disorder, describing the case of a girl with a heterozygous mutation in DNM1L and affected by progressive infantile encephalopathy, with cardiomyopathy and fatal paroxysmal vomiting correlated with bulbar transitory abnormal T2 hyperintensities and diffusion-weighted imaging (DWI) restriction areas, but without epilepsy. In patients with DNM1L mutations, careful evaluation for cardiac involvement is recommended.
Keywords: DNM1L; cardiomyopathy; mitochondrial disorders; mitochondrial dynamics; mitochondrial fission; paroxysmal vomiting.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Gerber S., Charif M., Chevrollier A., Chaumette T., Angebault C., Kane M.S., Paris A., Alban J., Quiles M., Delettre C., et al. Mutations in DNM1L, as in OPA1, result in dominant optic atrophy despite opposite effects on mitochondrial fusion and fission. Brain J. Neurol. 2017;140:2586–2596. doi: 10.1093/brain/awx219. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

