Joint Inflammation Correlates with Joint GPR30 Expression in Males and Hippocampal GPR30 Expression in Females in a Rat Model of Rheumatoid Arthritis
- PMID: 39063107
- PMCID: PMC11277240
- DOI: 10.3390/ijms25147864
Joint Inflammation Correlates with Joint GPR30 Expression in Males and Hippocampal GPR30 Expression in Females in a Rat Model of Rheumatoid Arthritis
Abstract
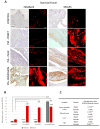
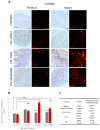
It is not entirely clear how the interaction between joint inflammation and the central nervous system (CNS) response in rheumatoid arthritis (RA) works, and what pathophysiology underlies the sex differences in coexisting neuropsychiatric comorbidities. It is known that estrogen hormones reduce inflammation in RA and that this occurs mainly via the stimulation of G protein-coupled receptor-30 (GPR30), also known as G protein-coupled estrogen receptor (GPER) 1. However, changes in GPR30 expression and sex differences induced by local and systemic inflammation in RA are not yet known. Our aim was to reveal sex differences in the expression and association of joint GPR30 with local and systemic inflammation, clinical course and furthermore with hippocampal GPR30 expression during pristane-induced arthritis (PIA) in Dark Agouti (DA) rats, an animal model of RA. Furthermore, we demonstrated sex-specific differences in the association between joint and systemic inflammation and hippocampal microglia during PIA. Our results suggest sex-specific differences not only in the clinical course and serum levels of pro-inflammatory cytokines but also in the expression of GPR30. Female rats show greater synovial inflammation and greater damage to the articular cartilage compared to males during PIA attack. Male rats express higher levels of synovial and cartilaginous GPR30 than females during PIA, which correlates with a less severe clinical course. The correlation between synovial and cartilaginous GPR30 and joint inflammation scores (Krenn and Mankin) in male rats suggests that the more severe the joint inflammation, the higher the GPR30 expression. At the same time, there is no particular upregulation of hippocampal GPR30 in males. On the other hand, female rats express higher levels of neuroprotective GPR30 in the hippocampus than male rats at the basic level and during PIA attack. In addition, females have a higher number of Iba-1+ cells in the hippocampus during PIA attack that strongly correlates with the clinical score, serum levels of IL-17A, and Krenn and Mankin scores. These results suggest that male rats are better protected from inflammation in the joints and female rats are better protected from the inflammation in the hippocampus during a PIA attack, independently of microglia proliferation. However, in the remission phase, synovial GPR30 expression suddenly increases in female rats, as does hippocampal GPR30 expression in males. Further experiments with a longer remission period are needed to investigate the molecular background of these sex differences, as well as microglia phenotype profiling.
Keywords: G protein-coupled estrogen receptor 1; Krenn synovitis score; Mankin osteoarthritis score; cytokines; hippocampus; microglia; neuroinflammation; rheumatoid arthritis.
Conflict of interest statement
Authors declare no conflict of interest.
Figures





References
-
- Matcham F., Norton S., Scott D.L., Steer S., Hotopf M. Symptoms of Depression and Anxiety Predict Treatment Response and Long-Term Physical Health Outcomes in Rheumatoid Arthritis: Secondary Analysis of a Randomized Controlled Trial. Rheumatology. 2015;55:268–278. doi: 10.1093/rheumatology/kev306. - DOI - PMC - PubMed
-
- Michelsen B., Kristianslund E.K., Sexton J., Hammer H.B., Fagerli K.M., Lie E., Wierød A., Kalstad S., Rødevand E., Krøll F., et al. Do Depression and Anxiety Reduce the Likelihood of Remission in Rheumatoid Arthritis and Psoriatic Arthritis? Data from the Prospective Multicentre NOR-DMARD Study. Ann. Rheum. Dis. 2017;76:1906–1910. doi: 10.1136/annrheumdis-2017-211284. - DOI - PubMed
-
- Giacometti J., Grubić-Kezele T. Olive Leaf Polyphenols Attenuate the Clinical Course of Experimental Autoimmune Encephalomyelitis and Provide Neuroprotection by Reducing Oxidative Stress, Regulating Microglia and SIRT1, and Preserving Myelin Integrity. Oxid. Med. Cell. Longev. 2020;2020:1–20. doi: 10.1155/2020/6125638. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

