Liver Fibrosis: From Basic Science towards Clinical Progress, Focusing on the Central Role of Hepatic Stellate Cells
- PMID: 39063116
- PMCID: PMC11277292
- DOI: 10.3390/ijms25147873
Liver Fibrosis: From Basic Science towards Clinical Progress, Focusing on the Central Role of Hepatic Stellate Cells
Abstract
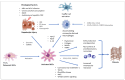
The burden of chronic liver disease is globally increasing at an alarming rate. Chronic liver injury leads to liver inflammation and fibrosis (LF) as critical determinants of long-term outcomes such as cirrhosis, liver cancer, and mortality. LF is a wound-healing process characterized by excessive deposition of extracellular matrix (ECM) proteins due to the activation of hepatic stellate cells (HSCs). In the healthy liver, quiescent HSCs metabolize and store retinoids. Upon fibrogenic activation, quiescent HSCs transdifferentiate into myofibroblasts; lose their vitamin A; upregulate α-smooth muscle actin; and produce proinflammatory soluble mediators, collagens, and inhibitors of ECM degradation. Activated HSCs are the main effector cells during hepatic fibrogenesis. In addition, the accumulation and activation of profibrogenic macrophages in response to hepatocyte death play a critical role in the initiation of HSC activation and survival. The main source of myofibroblasts is resident HSCs. Activated HSCs migrate to the site of active fibrogenesis to initiate the formation of a fibrous scar. Single-cell technologies revealed that quiescent HSCs are highly homogenous, while activated HSCs/myofibroblasts are much more heterogeneous. The complex process of inflammation results from the response of various hepatic cells to hepatocellular death and inflammatory signals related to intrahepatic injury pathways or extrahepatic mediators. Inflammatory processes modulate fibrogenesis by activating HSCs and, in turn, drive immune mechanisms via cytokines and chemokines. Increasing evidence also suggests that cellular stress responses contribute to fibrogenesis. Recent data demonstrated that LF can revert even at advanced stages of cirrhosis if the underlying cause is eliminated, which inhibits the inflammatory and profibrogenic cells. However, despite numerous clinical studies on plausible drug candidates, an approved antifibrotic therapy still remains elusive. This state-of-the-art review presents cellular and molecular mechanisms involved in hepatic fibrogenesis and its resolution, as well as comprehensively discusses the drivers linking liver injury to chronic liver inflammation and LF.
Keywords: hepatic stellate cells; hepatocytes; liver fibrosis; liver fibrosis resolution; liver sinusoidal endothelial cells; macrophages; myofibroblasts.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

