Profound Properties of Protein-Rich, Platelet-Rich Plasma Matrices as Novel, Multi-Purpose Biological Platforms in Tissue Repair, Regeneration, and Wound Healing
- PMID: 39063156
- PMCID: PMC11277244
- DOI: 10.3390/ijms25147914
Profound Properties of Protein-Rich, Platelet-Rich Plasma Matrices as Novel, Multi-Purpose Biological Platforms in Tissue Repair, Regeneration, and Wound Healing
Abstract
Autologous platelet-rich plasma (PRP) preparations are prepared at the point of care. Centrifugation cellular density separation sequesters a fresh unit of blood into three main fractions: a platelet-poor plasma (PPP) fraction, a stratum rich in platelets (platelet concentrate), and variable leukocyte bioformulation and erythrocyte fractions. The employment of autologous platelet concentrates facilitates the biological potential to accelerate and support numerous cellular activities that can lead to tissue repair, tissue regeneration, wound healing, and, ultimately, functional and structural repair. Normally, after PRP preparation, the PPP fraction is discarded. One of the less well-known but equally important features of PPP is that particular growth factors (GFs) are not abundantly present in PRP, as they reside outside of the platelet alpha granules. Precisely, insulin-like growth factor-1 (IGF-1) and hepatocyte growth factor (HGF) are mainly present in the PPP fraction. In addition to their roles as angiogenesis activators, these plasma-based GFs are also known to inhibit inflammation and fibrosis, and they promote keratinocyte migration and support tissue repair and wound healing. Additionally, PPP is known for the presence of exosomes and other macrovesicles, exerting cell-cell communication and cell signaling. Newly developed ultrafiltration technologies incorporate PPP processing methods by eliminating, in a fast and efficient manner, plasma water, cytokines, molecules, and plasma proteins with a molecular mass (weight) less than the pore size of the fibers. Consequently, a viable and viscous protein concentrate of functional total proteins, like fibrinogen, albumin, and alpha-2-macroglobulin is created. Consolidating a small volume of high platelet concentrate with a small volume of highly concentrated protein-rich PPP creates a protein-rich, platelet-rich plasma (PR-PRP) biological preparation. After the activation of proteins, mainly fibrinogen, the PR-PRP matrix retains and facilitates interactions between invading resident cells, like macrophages, fibroblast, and mesenchymal stem cells (MSCs), as well as the embedded concentrated PRP cells and molecules. The administered PR-PRP biologic will ultimately undergo fibrinolysis, leading to a sustained release of concentrated cells and molecules that have been retained in the PR-PRP matrix until the matrix is dissolved. We will discuss the unique biological and tissue reparative and regenerative properties of the PR-PRP matrix.
Keywords: fibrin(ogen); matrix; platelet-rich plasma; protein-rich platelet concentrate; tissue regeneration; tissue repair; ultrafiltration.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

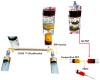

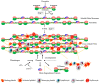

References
-
- Vallet-Regí M. Evolution of Biomaterials. Front. Mater. 2022;9:864016. doi: 10.3389/fmats.2022.864016. - DOI
-
- Todros S., Todesco M., Bagno A. Biomaterials and Their Biomedical Applications: From Replacement to Regeneration. Processes. 2021;9:1949. doi: 10.3390/pr9111949. - DOI
-
- Van Belleghem S.M., Mahadik B., Snodderly K.L., Fisher J.P. Biomaterials Science. Elsevier; Amsterdam, The Netherlands: 2020. Overview of tissue engineering concepts and applications; pp. 1289–1316.
-
- Van Blitterswijk C., De Boer J. Tissue Engineering. Academic Press; Cambridge, MA, USA: 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

