Cellular Senescence and Extracellular Vesicles in the Pathogenesis and Treatment of Obesity-A Narrative Review
- PMID: 39063184
- PMCID: PMC11276987
- DOI: 10.3390/ijms25147943
Cellular Senescence and Extracellular Vesicles in the Pathogenesis and Treatment of Obesity-A Narrative Review
Abstract
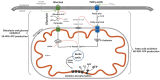
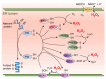
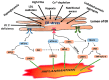
This narrative review explores the pathophysiology of obesity, cellular senescence, and exosome release. When exposed to excessive nutrients, adipocytes develop mitochondrial dysfunction and generate reactive oxygen species with DNA damage. This triggers adipocyte hypertrophy and hypoxia, inhibition of adiponectin secretion and adipogenesis, increased endoplasmic reticulum stress and maladaptive unfolded protein response, metaflammation, and polarization of macrophages. Such feed-forward cycles are not resolved by antioxidant systems, heat shock response pathways, or DNA repair mechanisms, resulting in transmissible cellular senescence via autocrine, paracrine, and endocrine signaling. Senescence can thus affect preadipocytes, mature adipocytes, tissue macrophages and lymphocytes, hepatocytes, vascular endothelium, pancreatic β cells, myocytes, hypothalamic nuclei, and renal podocytes. The senescence-associated secretory phenotype is closely related to visceral adipose tissue expansion and metaflammation; inhibition of SIRT-1, adiponectin, and autophagy; and increased release of exosomes, exosomal micro-RNAs, pro-inflammatory adipokines, and saturated free fatty acids. The resulting hypernefemia, insulin resistance, and diminished fatty acid β-oxidation lead to lipotoxicity and progressive obesity, metabolic syndrome, and physical and cognitive functional decline. Weight cycling is related to continuing immunosenescence and exposure to palmitate. Cellular senescence, exosome release, and the transmissible senescence-associated secretory phenotype contribute to obesity and metabolic syndrome. Targeted therapies have interrelated and synergistic effects on cellular senescence, obesity, and premature aging.
Keywords: AMPK; DDR; ER stress; NEFA; ROS; SASP; SIRT-1; VAT; Western-type diet; adiponectin; aging; autophagy; cellular senescence; epigenome; exosomes; extracellular vesicles; hypertrophic obesity; insulin resistance; lipotoxicity; metabolic syndrome; miRNA; obesogenic environment; p53; senolytic.
Conflict of interest statement
R.B.W. declares funding support for teaching, education and research from Ethicon and Fisher and Paykel. The other co-authors declare no conflict of interest.
Figures









References
-
- WHO Obesity and Overweight. [(accessed on 6 January 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

