Plastic Fly: What Drosophila melanogaster Can Tell Us about the Biological Effects and the Carcinogenic Potential of Nanopolystyrene
- PMID: 39063206
- PMCID: PMC11277132
- DOI: 10.3390/ijms25147965
Plastic Fly: What Drosophila melanogaster Can Tell Us about the Biological Effects and the Carcinogenic Potential of Nanopolystyrene
Abstract
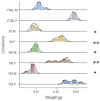
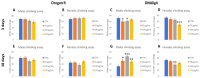
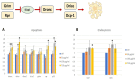
Today, plastic pollution is one of the biggest threats to the environment and public health. In the tissues of exposed species, micro- and nano-fragments accumulate, leading to genotoxicity, altered metabolism, and decreased lifespan. A model to investigate the genotoxic and tumor-promoting potential of nanoplastics (NPs) is Drosophila melanogaster. Here we tested polystyrene, which is commonly used in food packaging, is not well recycled, and makes up at least 30% of landfills. In order to investigate the biological effects and carcinogenic potential of 100 µm polystyrene nanoparticles (PSNPs), we raised Oregon [R] wild-type flies on contaminated food. After prolonged exposure, fluorescent PSNPs accumulated in the gut and fat bodies. Furthermore, PSNP-fed flies showed considerable alterations in weight, developmental time, and lifespan, as well as a compromised ability to recover from starvation. Additionally, we noticed a decrease in motor activity in DNAlig4 mutants fed with PSNPs, which are known to be susceptible to dietary stressors. A qPCR molecular investigation of the larval intestines revealed a markedly elevated expression of the genes drice and p53, suggesting a response to cell damage. Lastly, we used warts-defective mutants to assess the carcinogenic potential of PSNPs and discovered that exposed flies had more aberrant masses than untreated ones. In summary, our findings support the notion that ingested nanopolystyrene triggers metabolic and genetic modifications in the exposed organisms, eventually delaying development and accelerating death and disease.
Keywords: Drosophila melanogaster; carcinogenic nanoplastics; genotoxicity; in vivo models; nanopolystyrene.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- De Frond H., Hampton L.T., Kotar S., Gesulga K., Matuch C., Lao W., Weisberg S.B., Wong C.S., Rochman C.M. Monitoring microplastics in drinking water: An interlaboratory study to inform effective methods for quantifying and characterizing microplastics. Chemosphere. 2022;298:134282. doi: 10.1016/j.chemosphere.2022.134282. - DOI - PubMed
-
- Čerkasova N., Enders K., Lenz R., Oberbeckmann S., Brandt J., Fischer D., Fischer F., Labrenz M., Schernewski G. A Public Database for Microplastics in the Environment. Microplastics. 2023;2:132–146. doi: 10.3390/microplastics2010010. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

