Opioid Use and Gut Dysbiosis in Cancer Pain Patients
- PMID: 39063241
- PMCID: PMC11276997
- DOI: 10.3390/ijms25147999
Opioid Use and Gut Dysbiosis in Cancer Pain Patients
Abstract
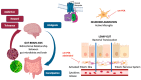
Opioids are commonly used for the management of severe chronic cancer pain. Their well-known pharmacological effects on the gastrointestinal system, particularly opioid-induced constipation (OIC), are the most common limiting factors in the optimization of analgesia, and have led to the wide use of laxatives and/or peripherally acting mu-opioid receptor antagonists (PAMORAs). A growing interest has been recently recorded in the possible effects of opioid treatment on the gut microbiota. Preclinical and clinical data, as presented in this review, showed that alterations of the gut microbiota play a role in modulating opioid-mediated analgesia and tolerability, including constipation. Moreover, due to the bidirectional crosstalk between gut bacteria and the central nervous system, gut dysbiosis may be crucial in modulating opioid reward and addictive behavior. The microbiota may also modulate pain regulation and tolerance, by activating microglial cells and inducing the release of inflammatory cytokines and chemokines, which sustain neuroinflammation. In the subset of cancer patients, the clinical meaning of opioid-induced gut dysbiosis, particularly its possible interference with the efficacy of chemotherapy and immunotherapy, is still unclear. Gut dysbiosis could be a new target for treatment in cancer patients. Restoring the physiological amount of specific gut bacteria may represent a promising therapeutic option for managing gastrointestinal symptoms and optimizing analgesia for cancer patients using opioids.
Keywords: PAMORAs; analgesia; constipation; gut dysbiosis; gut–brain axis; microbiota; neuroinflammation; opioids; tolerance.
Conflict of interest statement
Authors Joseph V. Pergolizzi and Jo Ann LeQuang were employed by the company NEMA Research. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Agulló L., Muriel J., Margarit C., Escorial M., Garcia D., Herrero M.J., Hervás D., Sandoval J., Peiró A.M. Sex Differences in Opioid Response Linked to OPRM1 and COMT genes DNA Methylation/Genotypes Changes in Patients with Chronic Pain. J. Clin. Med. 2023;12:3449. doi: 10.3390/jcm12103449. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

