Hydrogels for Neural Regeneration: Exploring New Horizons
- PMID: 39063768
- PMCID: PMC11278084
- DOI: 10.3390/ma17143472
Hydrogels for Neural Regeneration: Exploring New Horizons
Abstract
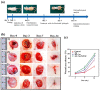
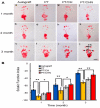
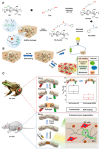
Nerve injury can significantly impair motor, sensory, and autonomic functions. Understanding nerve degeneration, particularly Wallerian degeneration, and the mechanisms of nerve regeneration is crucial for developing effective treatments. This manuscript reviews the use of advanced hydrogels that have been researched to enhance nerve regeneration. Hydrogels, due to their biocompatibility, tunable properties, and ability to create a supportive microenvironment, are being explored for their effectiveness in nerve repair. Various types of hydrogels, such as chitosan-, alginate-, collagen-, hyaluronic acid-, and peptide-based hydrogels, are discussed for their roles in promoting axonal growth, functional recovery, and myelination. Advanced formulations incorporating growth factors, bioactive molecules, and stem cells show significant promise in overcoming the limitations of traditional therapies. Despite these advancements, challenges in achieving robust and reliable nerve regeneration remain, necessitating ongoing research to optimize hydrogel-based interventions for neural regeneration.
Keywords: biocompatibility; clinical translation; hydrogels; neural regeneration; neural scaffolding.
Conflict of interest statement
Authors partly used the OpenAI Large-Scale Language Model to maximize accuracy, clarity, and organization.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

