ZnO:CuO Composites Obtained by Rapid Joule Heating for Photocatalysis
- PMID: 39063796
- PMCID: PMC11278348
- DOI: 10.3390/ma17143502
ZnO:CuO Composites Obtained by Rapid Joule Heating for Photocatalysis
Abstract
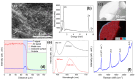
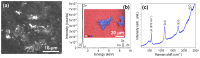
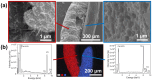
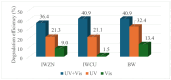
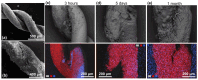
Semiconductor oxides belonging to various families are ideal candidates for application in photocatalytic processes. One of the challenges facing photocatalytic processes today is improving their efficiency under sunlight irradiation. In this study, the growth and characterization of semiconductor oxide nanostructures and composites based on the ZnO and CuO families are proposed. The selected growth method is the resistive heating of Zn and Cu wires to produce the corresponding oxides, combined with galvanic corrosion of Zn. An exhaustive characterization of the materials obtained has been carried out using techniques based on scanning electron microscopy and optical spectroscopies. The method we have followed and the conditions used in this study present promising results, not only from a degradation efficiency point of view but also because it is a cheap, easy, and fast growth method. These characteristics are essential in order to scale the process beyond the laboratory.
Keywords: Joule heating; copper oxide; galvanic corrosion; photocatalysis; zinc oxide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Sharma D.K., Shukla S., Sharma K.K., Kumar V. A Review on ZnO: Fundamental Properties and Applications. Mater. Today Proc. 2020;49:3028–3035. doi: 10.1016/j.matpr.2020.10.238. - DOI
-
- Comini E., Baratto C., Faglia G., Ferroni M., Ponzoni A., Zappa D., Sberveglieri G. Metal Oxide Nanowire Chemical and Biochemical Sensors. J. Mater. Res. 2013;28:2911–2931. doi: 10.1557/jmr.2013.304. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

