Novel Collagen Membrane Formulations with Irinotecan or Minocycline for Potential Application in Brain Cancer
- PMID: 39063802
- PMCID: PMC11278765
- DOI: 10.3390/ma17143510
Novel Collagen Membrane Formulations with Irinotecan or Minocycline for Potential Application in Brain Cancer
Abstract
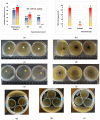
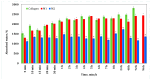
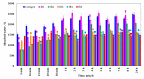
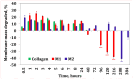
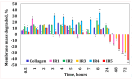
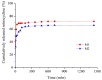
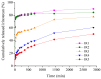
Our study explores the development of collagen membranes with integrated minocycline or irinotecan, targeting applications in tissue engineering and drug delivery systems. Type I collagen, extracted from bovine skin using advanced fibril-forming technology, was crosslinked with glutaraldehyde to create membranes. These membranes incorporated minocycline, an antibiotic, or irinotecan, a chemotherapeutic agent, in various concentrations. The membranes, varying in drug concentration, were studied by water absorption and enzymatic degradation tests, demonstrating a degree of permeability. We emphasize the advantages of local drug delivery for treating high-grade gliomas, highlighting the targeted approach's efficacy in reducing systemic adverse effects and enhancing drug bioavailability at the tumor site. The utilization of collagen membranes is proposed as a viable method for local drug delivery. Irinotecan's mechanism, a topoisomerase I inhibitor, and minocycline's broad antibacterial spectrum and inhibition of glial cell-induced membrane degradation are discussed. We critically examine the challenges posed by the systemic administration of chemotherapeutic agents, mainly due to the blood-brain barrier's restrictive nature, advocating for local delivery methods as a more effective alternative for glioblastoma treatment. These local delivery strategies, including collagen membranes, are posited as significant advancements in enhancing therapeutic outcomes for glioblastoma patients.
Keywords: bioengineering; collagen-based membranes; glioblastoma; irinotecan; minocycline; nanotechnology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
-
- Weller M., van den Bent M., Hopkins K., Tonn J.C., Stupp R., Falini A., Cohen-Jonathan-Moyal E., Frappaz D., Henriksson R., Balana C., et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15:e395–e403. doi: 10.1016/S1470-2045(14)70011-7. - DOI - PubMed
-
- Weller M., van den Bent M., Tonn J.C., Stupp R., Preusser M., Cohen-Jonathan-Moyal E., Henriksson R., Le Rhun E., Balana C., Chinot O., et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18:e315–e329. doi: 10.1016/S1470-2045(17)30194-8. - DOI - PubMed
-
- Soffietti R., Baumert B.G., Bello L., Von Deimling A., Duffau H., Frénay M., Grisold W., Grant R., Graus F., Hoang-Xuan K., et al. Guidelines on management of low-grade gliomas: Report of an EFNS-EANO Task Force. Eur. J. Neurol. 2010;17:1124–1133. doi: 10.1111/j.1468-1331.2010.03151.x. - DOI - PubMed
-
- Wen P.Y., Macdonald D.R., Reardon D.A., Cloughesy T.F., Sorensen A.G., Galanis E., Degroot J., Wick W., Gilbert M.R., Lassman A.B., et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

