Synthetic Genitourinary Image Synthesis via Generative Adversarial Networks: Enhancing Artificial Intelligence Diagnostic Precision
- PMID: 39063957
- PMCID: PMC11278131
- DOI: 10.3390/jpm14070703
Synthetic Genitourinary Image Synthesis via Generative Adversarial Networks: Enhancing Artificial Intelligence Diagnostic Precision
Abstract
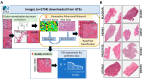
Introduction: In the realm of computational pathology, the scarcity and restricted diversity of genitourinary (GU) tissue datasets pose significant challenges for training robust diagnostic models. This study explores the potential of Generative Adversarial Networks (GANs) to mitigate these limitations by generating high-quality synthetic images of rare or underrepresented GU tissues. We hypothesized that augmenting the training data of computational pathology models with these GAN-generated images, validated through pathologist evaluation and quantitative similarity measures, would significantly enhance model performance in tasks such as tissue classification, segmentation, and disease detection.
Methods: To test this hypothesis, we employed a GAN model to produce synthetic images of eight different GU tissues. The quality of these images was rigorously assessed using a Relative Inception Score (RIS) of 1.27 ± 0.15 and a Fréchet Inception Distance (FID) that stabilized at 120, metrics that reflect the visual and statistical fidelity of the generated images to real histopathological images. Additionally, the synthetic images received an 80% approval rating from board-certified pathologists, further validating their realism and diagnostic utility. We used an alternative Spatial Heterogeneous Recurrence Quantification Analysis (SHRQA) to assess the quality of prostate tissue. This allowed us to make a comparison between original and synthetic data in the context of features, which were further validated by the pathologist's evaluation. Future work will focus on implementing a deep learning model to evaluate the performance of the augmented datasets in tasks such as tissue classification, segmentation, and disease detection. This will provide a more comprehensive understanding of the utility of GAN-generated synthetic images in enhancing computational pathology workflows.
Results: This study not only confirms the feasibility of using GANs for data augmentation in medical image analysis but also highlights the critical role of synthetic data in addressing the challenges of dataset scarcity and imbalance.
Conclusions: Future work will focus on refining the generative models to produce even more diverse and complex tissue representations, potentially transforming the landscape of medical diagnostics with AI-driven solutions.
Keywords: GAN; diagnostics; digital imaging; machine learning; pathology; prognostics; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Fabijan A., Zawadzka-Fabijan A., Fabijan R., Zakrzewski K., Nowoslawska E., Polis B. Artificial Intelligence in Medical Imaging: Analyzing the Performance of ChatGPT and Microsoft Bing in Scoliosis Detection and Cobb Angle Assessment. Diagnostics. 2024;14:773. doi: 10.3390/diagnostics14070773. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

