Retention Rate of Ixekizumab in Psoriatic Arthritis: A Real-World Study
- PMID: 39063970
- PMCID: PMC11278385
- DOI: 10.3390/jpm14070716
Retention Rate of Ixekizumab in Psoriatic Arthritis: A Real-World Study
Abstract
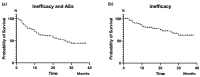
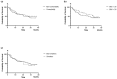
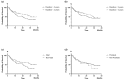
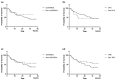
We aimed to examine the drug retention rate (DRR) of the interleukin-17 inhibitor ixekizumab in a real-world monocentric cohort of psoriatic arthritis (PsA) patients and to assess the predictors of drug discontinuation. Consecutive PsA patients who underwent treatment with ixekizumab from October 2019 to February 2023 were enrolled in this observational, retrospective, monocentric study. Clinical records were assessed at baseline and throughout the follow-up period. We collected sociodemographic data, smoking habits, body mass index, the presence of Human Leukocyte Antigen B27, comorbidities, disease involvement and duration, previous therapy, discontinuation of ixekizumab, reasons for discontinuation, and adverse events (AEs). DRR was evaluated as time to drug discontinuation and assessed through Kaplan-Meier curves. Baseline factors predicting drug discontinuation were investigated through logistic regression models. Eighty PsA patients were included in this study. Ixekizumab was administered at a dose of 160 mg by subcutaneous injection at baseline, followed by 80 mg every four weeks thereafter. Ixekizumab had a 38-month-cumulative DRR of 43.8%, accounting for both inefficacy and AEs. When considering only inefficacy, the DRR was 62.6%. Comorbidities (p = 0.665), obesity (p = 0.665), smoking (p = 0.884), disease duration ≤ 2 years (p = 0.071), axial (p = 0.131) and skin involvement (p = 0.460), and previous therapies, including conventional synthetic (p = 0.504) and biological (p = 0.474) Disease-Modifying Antirheumatic Drugs (bDMARDs), as well as the number of previous bDMARDs or targeted synthetic Disease-Modifying Antirheumatic Drugs (tsDMARDs), did not significantly affect the DRR (p = 0.349). Multivariate analysis found no independent predictors of drug discontinuation. The most frequent AEs leading to discontinuation were skin reactions; no severe infections were observed. In our real-world study, comorbidities, disease duration, and previous therapies did not affect the DRR of ixekizumab. Ixekizumab had a favorable safety profile, with no severe AEs observed.
Keywords: drug retention rate; ixekizumab; psoriatic arthritis.
Conflict of interest statement
The authors declare the following conflicts of interest. E.B.: Honoraria: BMS, Novartis. P.R.: Honoraria: Abbvie, BMS, Ely Lilly, Janssen Novartis, Sobi; educational grants: BMS; research support: Pfizer. D.D.: Honoraria: Janssen. G.C.: advisory boards: Novartis, AbbVie, Alfa-sigma; speakers’ bureau: Janssen, Galapagos, Eli-Lilly, BMS. V.D.: no conflict of interest. M. Gammino: no conflict of interest. M. Gatto: Honoraria, speakers bureau from: GSK, Astrazeneca, Janssen. V.G.: no conflict of interest. C.L.: Honoraria, advisory boards, speakers’ bureau, educational grants, research support from: BMS, Pfizer, AbbVie, Janssen, Eli-Lilly. E.M.: no conflict of interest; M.S.: no conflict of interest. A.I.: Honoraria, advisory boards, speakers’ bureau, educational grants, research support from: AbbVie, Alfa-sigma, BMS, Celgene, Celltrion, Eli-Lilly, Galapagos, Gilead, MSD, Janssen, Novartis, Pfizer, Sanofi Genzyme, SOBI.
Figures
References
-
- Naredo E., Moller I., De Miguel E., Batlle-Gualda E., Acebes C., Brito E., Mayordomo L., Moragues C., Uson J., De Agustin J.J., et al. High Prevalence of Ultrasonographic Synovitis and Enthesopathy in Patients with Psoriasis without Psoriatic Arthritis: A Prospective Case-Control Study. Rheumatology. 2011;50:1838–1848. doi: 10.1093/rheumatology/ker078. - DOI - PubMed
-
- Zuliani F., Zabotti A., Errichetti E., Tinazzi I., Zanetti A., Carrara G., Quartuccio L., Sacco S., Giovannini I., Stinco G., et al. Ultrasonographic Detection of Subclinical Enthesitis and Synovitis: A Possible Stratification of Psoriatic Patients without Clinical Musculoskeletal Involvement. Clin. Exp. Rheumatol. 2019;37:593–599. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

