Dupilumab Improves Facial Pain and Reduces Rescue Treatments in Patients with CRSwNP and Recalcitrant Frontal Sinusitis
- PMID: 39063989
- PMCID: PMC11277593
- DOI: 10.3390/jpm14070735
Dupilumab Improves Facial Pain and Reduces Rescue Treatments in Patients with CRSwNP and Recalcitrant Frontal Sinusitis
Abstract
Recalcitrant frontal sinusitis in patients with chronic rhinosinusitis and nasal polyps (CRSwNP) has a negative impact on their quality of life due to frontal pain and a high risk of sinus occlusion, thus necessitating antibiotics, systemic corticosteroids, and multiple surgeries. The aim of this study was to assess the efficacy of dupilumab in reducing frontal pain and the need for rescue treatments for recalcitrant frontal sinusitis in patients with CRSwNP. We enrolled a cohort of 10 patients with severe uncontrolled CRSwNP and concomitant recurrent frontal sinusitis associated with severe facial pain measured by MIDAS score who were treated with dupilumab 300 mg every 2 weeks and followed for at least 12 months. The mean MIDAS score decreased from 45.6 ± 10.7 at baseline to 1.3 ± 2.3 at 6 months (p < 0.05). VAS craniofacial pain decreased from 7.3 ± 1.6 at baseline to 1.2 ± 1.5 at 6 months (p < 0.05). No patient needed oral corticosteroids during treatment with dupilumab (p < 0.05), and the use of analgesics decreased from 9.6 ± 3.1 NSAID pills/week in the last 2 months at baseline to 0.6 ± 1.3 at 1 year of follow-up (p < 0.05). Our results demonstrated that use of subcutaneous dupilumab can improve symptom control, including recurrent severe cranio-facial pain, and reduce the need for rescue medical treatments (systemic steroids and NSAID) in patients with severe uncontrolled CRSwNP and concomitant recurrent frontal sinusitis.
Keywords: biologics; chronic rhinosinusitis; endoscopic sinus surgery; frontal sinusitis; headache; nasal polyps; oral corticosteroids.
Conflict of interest statement
E.D.C.: Lecture fees and participations on expert board meeting of GSK, Novartis, Sanofi, Firma, AstraZeneca. S.S., M.C. and C.M.: Lecture fees for GSK. D.P., G.D., M.R., A.R. (Alberta Rizzuti), M.C.P., T.D.C., S.L.V., A.R. (Angela Rizzi), R.C. and J.G. have no conflicts of interest.
Figures

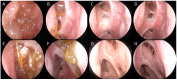
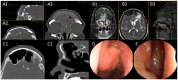
References
-
- De Corso E., Bellocchi G., De Benedetto M., Lombardo N., Macchi A., Malvezzi L., Motta G., Pagella F., Vicini C., Passali D. Biologics for Severe Uncontrolled Chronic Rhinosinusitis with Nasal Polyps: A Change Management Approach. Consensus of the Joint Committee of Italian Society of Otorhinolaryngology on Biologics in Rhinology. Acta Otorhinolaryngol. Ital. 2022;42:1–16. doi: 10.14639/0392-100X-N1614. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

