Retro Mode Imaging for Detection and Quantification of Sub-RPE Drusen and Subretinal Drusenoid Deposits in Age-Related Macular Degeneration
- PMID: 39064170
- PMCID: PMC11278487
- DOI: 10.3390/jcm13144131
Retro Mode Imaging for Detection and Quantification of Sub-RPE Drusen and Subretinal Drusenoid Deposits in Age-Related Macular Degeneration
Abstract
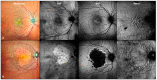
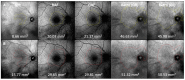
Background: Drusen and drusenoid deposits are a hallmark of age-related macular degeneration (AMD). Nowadays, a multimodal retinal imaging approach enables the detection of these deposits. However, quantitative data on subretinal drusenoid deposits (SDDs) are still missing. Here, we compare the capability of en-face drusen and SDD area detection in eyes with non-exudative AMD using conventional imaging modalities versus Retro mode imaging. We also quantitatively assess the topographic distribution of drusen and SDDs. Methods: In total, 120 eyes of 90 subjects (mean age ± standard deviation = 74.6 ± 8.6 years) were included. Coherent en-face drusen and SDD areas were measured via near-infrared reflectance, green (G-) and blue (B-) fundus autofluorescence (AF), and Retro mode imaging. Drusen phenotypes were classified by correlating en-face drusen areas using structural high-resolution spectral domain optical coherence tomography. The topographic distribution of drusen was analyzed according to a modified ETDRS (Early Treatment of Diabetic Retinopathy Study) grid. Intraclass correlation coefficient (ICC) analysis was applied to determine the inter-reader agreement in the SDD en-face area assessment. Results: The largest coherent en-face drusen area was found using Retro mode imaging with a mean area of 105.2 ± 45.9 mm2 (deviated left mode (DL)) and 105.4 ± 45.5 mm2 (deviated right mode (DR)). The smallest en-face drusen areas were determined by GAF (50.9 ± 42.6 mm2) and BAF imaging (49.1 ± 42.9 mm2) (p < 0.001). The inter-reader agreement for SDD en-face areas ranged from 0.93 (DR) to 0.70 (BAF). The topographic analysis revealed the highest number of SDDs in the superior peripheral retina, whereas sub-retinal pigment epithelium drusen were mostly found in the perifoveal retina. Retro mode imaging further enabled the detection of the earliest SDD stages. Conclusions: Retro mode imaging allows for a detailed detection of drusen phenotypes. While hundreds/thousands of SDDs can be present in one eye, the impact of SDD number or volume on AMD progression still needs to be evaluated. However, this new imaging modality can add important knowledge on drusen development and the pathophysiology of AMD.
Keywords: age-related macular degeneration; drusen; imaging; retro mode; subretinal drusenoid deposits.
Conflict of interest statement
Thomas Ach has received support by NIDEK, Japan by providing the retinal imaging device. The sponsors had no role in the design, execution, interpretation, or writing of the study. The other authors declare that this research was conducted in abscence of any commercial or financial relationship that could be contrued as a potential conflict of interest for this work.
Figures




Similar articles
-
Prevalence of Subretinal Drusenoid Deposits in Older Persons with and without Age-Related Macular Degeneration, by Multimodal Imaging.Ophthalmology. 2016 May;123(5):1090-100. doi: 10.1016/j.ophtha.2015.12.034. Epub 2016 Feb 10. Ophthalmology. 2016. PMID: 26875000 Free PMC article.
-
Quantification of Early and Intermediate Age-related Macular Degeneration Using OCT "en face" Presentation.Klin Monbl Augenheilkd. 2022 Jan;239(1):79-85. doi: 10.1055/a-1327-3633. Epub 2021 Jan 29. Klin Monbl Augenheilkd. 2022. PMID: 33513622 English, German.
-
En Face Optical Coherence Tomography Illustrates the Trizonal Distribution of Drusen and Subretinal Drusenoid Deposits in the Macula.Am J Ophthalmol. 2024 May;261:187-198. doi: 10.1016/j.ajo.2023.12.013. Epub 2024 Jan 12. Am J Ophthalmol. 2024. PMID: 38218515
-
Subretinal drusenoid deposits: An update.Taiwan J Ophthalmol. 2022 May 26;12(2):138-146. doi: 10.4103/tjo.tjo_18_22. eCollection 2022 Apr-Jun. Taiwan J Ophthalmol. 2022. PMID: 35813798 Free PMC article. Review.
-
Subretinal drusenoid deposits AKA pseudodrusen.Surv Ophthalmol. 2018 Nov-Dec;63(6):782-815. doi: 10.1016/j.survophthal.2018.05.005. Epub 2018 May 31. Surv Ophthalmol. 2018. PMID: 29859199 Review.
Cited by
-
En Face OCT and the Phenotype Characterization of Drusen.Invest Ophthalmol Vis Sci. 2025 Jul 1;66(9):52. doi: 10.1167/iovs.66.9.52. Invest Ophthalmol Vis Sci. 2025. PMID: 40679333 Free PMC article.
-
Quantifications of Outer Retinal Bands in Geographic Atrophy by Comparing Superior Axial Resolution and Conventional OCT.Invest Ophthalmol Vis Sci. 2025 Apr 1;66(4):65. doi: 10.1167/iovs.66.4.65. Invest Ophthalmol Vis Sci. 2025. PMID: 40261660 Free PMC article.
References
-
- VanDenLangenberg A.M., Carson M.P. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2023. Drusen Bodies. - PubMed
-
- Hageman G.S., Luthert P.J., Victor Chong N.H., Johnson L.V., Anderson D.H., Mullins R.F. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 2001;20:705–732. doi: 10.1016/S1350-9462(01)00010-6. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

