Structural Evolution of Small-Sized Phosphorus-Doped Boron Clusters: A Half-Sandwich-Structured PB15 Cluster
- PMID: 39064962
- PMCID: PMC11280394
- DOI: 10.3390/molecules29143384
Structural Evolution of Small-Sized Phosphorus-Doped Boron Clusters: A Half-Sandwich-Structured PB15 Cluster
Abstract
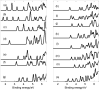
The present study is a theoretical investigation into the structural evolution, electronic properties, and photoelectron spectra of phosphorus-doped boron clusters PBn0/- (n = 3-17). The results of this study revealed that the lowest energy structures of PBn- (n = 3-17) clusters, except for PB17-, exhibit planar or quasi-planar structures. The lowest energy structures of PBn (n = 3-17), with the exceptions of PB7, PB9, and PB15, are planar or quasi-planar. The ground state of PB7 has an umbrella-shaped structure, with C6V symmetry. Interestingly, the neutral cluster PB15 has a half-sandwich-like structure, in which the P atom is attached to three B atoms at one end of the sandwich, exhibiting excellent relative and chemical stability due to its higher second-order energy difference and larger HOMO-LUMO energy gap of 4.31 eV. Subsequently, adaptive natural density partitioning (AdNDP) and electron localization function (ELF) analyses demonstrate the bonding characteristics of PB7 and PB15, providing support for the validity of their stability. The calculated photoelectron spectra show distinct characteristic peaks of PBn- (n = 3-17) clusters, thus providing theoretical evidence for the future identification of doped boron clusters. In summary, our work has significant implications for understanding the structural evolution of doped boron clusters PBn0/- (n = 3-17), motivating further experiments regarding doped boron clusters.
Keywords: boron clusters; geometrical structures; phosphorus-doped; spectra.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures







References
-
- Das S., Shareef M.A., Das B.C. Design and Synthesis of New Boron-Based Benzo[c][1,2,5] oxadiazoles and Benzo[c][1,2,5] thiadiazoles as Potential Hypoxia Inhibitors. Inorganics. 2023;11:34. doi: 10.3390/inorganics11010034. - DOI
-
- Sivaev I.B., Bregadze V.I. Borane, Carborane and Metallacarborane Anions for Stabilization of Transient and Highly Reactive Intermediates. Volume 1. World Scientific; Singapore: 2018. pp. 147–203.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

