Microbiological and Imaging-Based Evaluations of Photodynamic Therapy Combined with Er:YAG Laser Therapy in the In Vitro Decontamination of Titanium and Zirconia Surfaces
- PMID: 39065113
- PMCID: PMC11278944
- DOI: 10.3390/microorganisms12071345
Microbiological and Imaging-Based Evaluations of Photodynamic Therapy Combined with Er:YAG Laser Therapy in the In Vitro Decontamination of Titanium and Zirconia Surfaces
Abstract
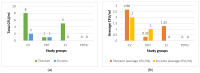
The oral cavity's soft and hard tissues create a conducive environment for microbial proliferation and biofilm development, facilitating the colonization of prosthodontic and implant materials such as titanium (Ti) and zirconia (Zr). This study aimed to compare the efficacy of conventional decontamination methodologies (i.e., chemical and mechanical, using 0.12% digluconate chlorhexidine (CHX) solution-treatment and airflow) to adjunctive laser-based interventions on Ti and Zr substrates inoculated with Staphylococcus (S.) aureus ATCC 25923. Additionally, this investigation sought to elucidate the impact of these treatments on temperature variations and surface integrity, analyzing the laser irradiation effects on these prevalent dental materials. Experimental configurations were delineated for both Ti and Zr samples across four groups: (1) a conventional treatment group (CV); (2) a photodynamic therapy group (PDT); (3) an Er:YAG laser treatment group (Er); (4) a combined PDT and Er:YAG treatment group (PDTEr). Also, a negative control group (C) that received no treatment was considered. The decontamination of the inoculated disc samples was evaluated by quantifying the microbial colonies in colony-forming units per milliliter (CFU/mL). Temperature variations on the surface of the samples were determined during laser treatments. Surface modifications were investigated using scanning electron microscopy (SEM) and optical coherence tomography (OCT). For statistical analysis, Fisher 95% confidence intervals, Hsu's MCB method, and the Kruskal-Wallis test were applied. With regard to the 105 CFU/mL of the negative control group, results indicated average values equal for each study group to (1) 2.66 CFU/mL for Ti and 2 CFU/mL for Zr for the CV group; (2) 0.33 CFU/mL for Ti and 1 CFU/mL for Zr for the PDT group; (3) 1.25 CFU/mL for Ti and 0 CFU/mL for Zr for the Er group; (4), and 0 CFU/mL for both Ti and Zr for the PDTEr group. Therefore, the combined PDT and Er:YAG treatment (PDTEr) and the singular PDT modality outperformed conventional decontamination methods in eradicating S. aureus biofilms from both Ti and Zr surfaces. Notably, the PDTEr regime achieved a comprehensive elimination of microbial colonies on treated substrates. Surface examination employing OCT demonstrated discernible alterations in the surface morphology of samples subjected to Er:YAG and combined PDT and Er:YAG treatments. Temperature checks during treatments showed no major changes, suggesting the applied laser methods are safe. In conclusion, PDTEr and PDT eliminated bacteria more effectively, but Zr surfaces were more resilient, making them better for microbe-controlling applications. Also, the study demonstrated that the (less costly but lower resolution) OCT method can replace SEM for such investigations.
Keywords: Er:YAG laser; Staphylococcus aureus; biofilm; dental implants; laser therapy; optical coherence tomography; photodynamic therapy; scanning electron microscopy; titanium; zirconia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



















References
-
- Øilo M., Bakken V. Biofilm and Dental Biomaterials. Materials. 2015;8:2887–2900. doi: 10.3390/ma8062887. - DOI
-
- Colombo A.P., Boches S.K., Cotton S.L., Goodson J.M., Kent R., Haffajee A.D., Socransky S.S., Hasturk H., Van Dyke T.E., Dewhirst F., et al. Comparisons of Subgingival Microbial Profiles of Refractory Periodontitis, Severe Periodontitis, and Periodontal Health Using the Human Oral Microbe Identification Microarray. J. Periodontol. 2009;80:1421–1432. doi: 10.1902/jop.2009.090185. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

