Poly Lactic-co-Glycolic Acid (PLGA) Loaded with a Squaraine Dye as Photosensitizer for Antimicrobial Photodynamic Therapy
- PMID: 39065279
- PMCID: PMC11281082
- DOI: 10.3390/polym16141962
Poly Lactic-co-Glycolic Acid (PLGA) Loaded with a Squaraine Dye as Photosensitizer for Antimicrobial Photodynamic Therapy
Abstract
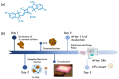
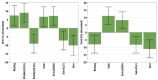
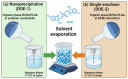
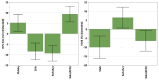
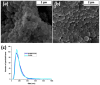
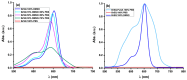
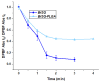
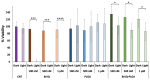
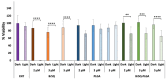
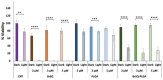
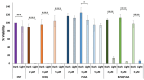
Antimicrobial Photodynamic Therapy (aPDT) is an innovative and promising method for combating infections, reducing the risk of antimicrobial resistance compared to traditional antibiotics. Squaraine (SQ) dyes can be considered promising photosensitizers (PSs) but are generally hydrophobic molecules that can self-aggregate under physiological conditions. To overcome these drawbacks, a possible solution is to incorporate SQs inside nanoparticles (NPs). The present work deals with the design and development of innovative nanophotosensitizers based on poly lactic-co-glycolic acid (PLGA) NPs incorporating a brominated squaraine (BrSQ) with potential application in aPDT. Two designs of experiments (DoEs) based on the single emulsion and nanoprecipitation methods were set up to investigate how different variables (type of solvent, solvent ratio, concentration of PLGA, stabilizer and dye, sonication power and time) can affect the size, zeta (ζ)-potential, yield, entrapment efficiency, and drug loading capacity of the SQ-PLGA NPs. SQ-PLGA NPs were characterized by NTA, FE-SEM, and UV-Vis spectroscopy and the ability to produce reactive oxygen species (ROS) was evaluated, proving that ROS generation ability is preserved in SQ-PLGA. In vitro antimicrobial activity against Gram-positive bacteria in planktonic state using Staphylococcus aureus was conducted in different conditions and pH to evaluate the potential of these nanophotosensitizers for aPDT in the local treatment of infections.
Keywords: Antimicrobial Photodynamic Therapy; Gram-positive bacteria; PLGA nanoparticles; designs of experiments; squaraine dyes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Uddin T.M., Chakraborty A.J., Khusro A., Zidan B.R.M., Mitra S., Bin Emran T., Dhama K., Ripon M.K.H., Gajdács M., Sahibzada M.U.K., et al. Antibiotic Resistance in Microbes: History, Mechanisms, Therapeutic Strategies and Future Prospects. J. Infect. Public Health. 2021;14:1750–1766. doi: 10.1016/j.jiph.2021.10.020. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

