Sulfonated Pentablock Copolymer (NexarTM) for Water Remediation and Other Applications
- PMID: 39065326
- PMCID: PMC11280590
- DOI: 10.3390/polym16142009
Sulfonated Pentablock Copolymer (NexarTM) for Water Remediation and Other Applications
Abstract
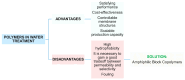
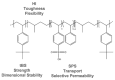
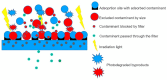
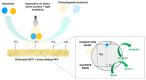
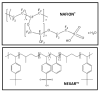
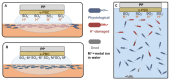
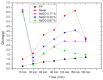
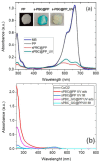
This review focuses on the use of a sulfonated pentablock copolymer commercialized as NexarTM in water purification applications. The properties and the use of sulfonated copolymers, in general, and of NexarTM, in particular, are described within a brief reference focusing on the problem of different water contaminants, purification technologies, and the use of nanomaterials and nanocomposites for water treatment. In addition to desalination and pervaporation processes, adsorption and photocatalytic processes are also considered here. The reported results confirm the possibility of using NexarTM as a matrix for embedded nanoparticles, exploiting their performance in adsorption and photocatalytic processes and preventing their dispersion in the environment. Furthermore, the reported antimicrobial and antibiofouling properties of NexarTM make it a promising material for achieving active coatings that are able to enhance commercial filter lifetime and performance. The coated filters show selective and efficient removal of cationic contaminants in filtration processes, which is not observed with a bare commercial filter. The UV surface treatment and/or the addition of nanostructures such as graphene oxide (GO) flakes confer NexarTM with coating additional functionalities and activity. Finally, other application fields of this polymer are reported, i.e., energy and/or gas separation, suggesting its possible use as an efficient and economical alternative to the more well-known Nafion polymer.
Keywords: NexarTM; adsorption; antibiofouling; coating; energy; filtration; photocatalysis; sulfonated copolymer; water.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures












References
-
- Zou C., Ma F., Pan S., Lin M., Zhang G., Xiong B., Wang Y., Liang Y., Yang Z. Earth energy evolution; human development and carbon neutral strategy. Petrol. Explor. Dev. 2022;49:468–488. doi: 10.1016/S1876-3804(22)60040-5. - DOI
-
- Gleeson T., Befus K., Jasechko S., Luijendijk E., Bayani Cardenas M. The global volume and distribution of modern groundwater. Nat. Geosci. 2016;9:161–167. doi: 10.1038/ngeo2590. - DOI
-
- Bashir I., Lone F.A., Bhat R.A., Mir S.A., Dar Z.A., Dar S.A. Concerns and Threats of Contamination on Aquatic Ecosystems. In: Hakeem K., Bhat R., Qadri H., editors. Bioremediation and Biotechnology. Springer; Cham, Switzerland: 2022. - DOI
-
- Zamora-Ledezma C., Negrete-Bolagay D., Figueroa F., Zamora-Ledezma E., Ni M., Alexis F., Guerrero V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021;22:101504. doi: 10.1016/j.eti.2021.101504. - DOI
-
- Boretti A., Rosa L. Reassessing the projections of the World Water Development Report. NPJ Clean Water. 2019;2:15. doi: 10.1038/s41545-019-0039-9. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

