Effects of Phloretin on Seedling Growth and Histochemical Distribution of Phenols, Polysaccharides and Lipids in Capsella bursa-pastoris (L.) Medik
- PMID: 39065417
- PMCID: PMC11280091
- DOI: 10.3390/plants13141890
Effects of Phloretin on Seedling Growth and Histochemical Distribution of Phenols, Polysaccharides and Lipids in Capsella bursa-pastoris (L.) Medik
Abstract
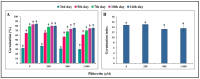
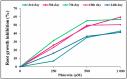
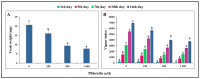
The present study evaluates the phytotoxic effects of phloretin, a prevalent secondary metabolite of apple trees, on the broadleaf weed Capsella bursa-pastoris (L.) Medik. known for its resistant myxospermous seeds that form a long-lasting soil bank. The results indicate a significant, dose-dependent inhibitory effect of phloretin on the growth and morphological parameters of weed seedlings grown in vitro. Although the applied phloretin concentrations (250-1000 µM) were not lethal to the C. bursa-pastoris seedlings after two weeks, the metabolism of the seedlings was impaired, resulting in an accumulation of lipid droplets in the root tips and root hairs. Histochemical analysis shows deposits of phenols in the root epidermal cells, which are probably aggregates of phloretin or its metabolic derivatives. The accumulation of pectin in the cell walls of root border cells in phloretin-treated seedlings indicates an attempt to reduce the uptake of phloretin and reduce its concentration in the cells. Inhibition of shoot growth associated with chlorosis and reduced photosynthetic pigment content is a consequence of seedling exposure to phloretin. This study provides a basis for further evaluation of phloretin as a new bioherbicidal compound and for elucidating the mechanism underlying its phytotoxic activity.
Keywords: Malus; allelopathy; apple; chlorophyll; dihydrochalcones; pectin; phytotoxic effect; starch.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Kostina-Bednarz M., Płonka J., Barchanska H. Allelopathy as a source of bioherbicides: Challenges and prospects for sustainable agriculture. Rev. Environ. Sci. Biotechnol. 2023;22:471–504. doi: 10.1007/s11157-023-09656-1. - DOI
-
- Dugé de Bernoville T., Gaucher M., Guyot S., Durel C.E., Dat J.F., Brisset M.N. The constitutive phenolic composition of two Malus × domestica genotypes is not responsible for their contrasted susceptibilities to fire blight. Environ. Exp. Bot. 2011;74:65–73. doi: 10.1016/j.envexpbot.2011.04.019. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

