Functional Mechanisms of Dietary Crocin Protection in Cardiovascular Models under Oxidative Stress
- PMID: 39065537
- PMCID: PMC11280316
- DOI: 10.3390/pharmaceutics16070840
Functional Mechanisms of Dietary Crocin Protection in Cardiovascular Models under Oxidative Stress
Abstract
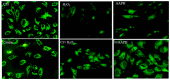
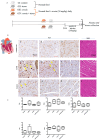
It was previously reported that crocin, a water-soluble carotenoid isolated from the Crocus sativus L. (saffron), has protective effects on cardiac cells and may neutralize and even prevent the formation of excess number of free radicals; however, functional mechanisms of crocin activity have been poorly understood. In the present research, we aimed to study the functional mechanism of crocin in the heart exposed to oxidative stress. Accordingly, oxidative stress was modeled in vitro on human umbilical vein endothelial cells (HUVECs) and in vivo in mice using cellular stressors. The beneficial effects of crocin were investigated at cellular and molecular levels in HUVECs and mice hearts. Results indicated that oral administration of crocin could have protective effects on HUVECs. In addition, it protects cardiac cells and significantly inhibits inflammation via modulating molecular signaling pathways TLR4/PTEN/AKT/mTOR/NF-κB and microRNA (miR-21). Here we show that crocin not only acts as a direct free radical scavenger but also modifies the gene expression profiles of HUVECs and protects mice hearts with anti-inflammatory action under oxidative stress.
Keywords: cardiovascular; crocin; dietary antioxidants; microRNA; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Krylatov A.V., Maslov L.N., Voronkov N.S., Boshchenko A.A., Popov S.V., Gomez L., Wang H., Jaggi A.S., Downey J.M. Reactive Oxygen Species as Intracellular Signaling Molecules in the Cardiovascular System. Curr. Cardiol. Rev. 2018;14:290–300. doi: 10.2174/1573403X14666180702152436. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

