Optimization of Diclofenac-Loaded Bicomponent Nanofibers: Effect of Gelatin on In Vitro and In Vivo Response
- PMID: 39065622
- PMCID: PMC11279899
- DOI: 10.3390/pharmaceutics16070925
Optimization of Diclofenac-Loaded Bicomponent Nanofibers: Effect of Gelatin on In Vitro and In Vivo Response
Abstract
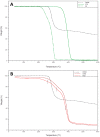
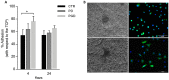
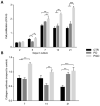
The use of electrospun fibers as anti-inflammatory drug carriers is currently one of the most interesting approaches for the design of drug delivery systems. In recent years, biodegradable polymers blended with naturally derived ones have been extensively studied to fabricate bioinspired platforms capable of driving biological responses by releasing selected molecular/pharmaceutical signals. Here, sodium diclofenac (DicNa)-loaded electrospun fibers, consisting of polycaprolactone (PCL) or gelatin-functionalized PCL, were studied to evaluate fibroblasts' in vitro and in vivo response. In vitro studies demonstrated that cell adhesion of L929 cells (≈70%) was not affected by the presence of DicNa after 4 h. Moreover, the initial burst release of the drug from PD and PGD fibers, e.g., 80 and 48%, respectively, after 5 h-combined with its sustained release-did not produce any cytotoxic effect and did not negatively influence the biological activity of the cells. In particular, it was demonstrated that the addition of gelatin concurred to slow down the release mechanism, thus limiting the antiproliferative effect of DicNa, as confirmed by the significant increase in cell viability and collagen deposition after 7 days, with respect to PCL alone. In vivo studies in a rat subcutaneous model also confirmed the ability of DicNa-loaded fibers to moderate the inflammatory/foreign body response independently through the presence of gelatin that played a significant role in supporting the formation of small-caliber vessels after 10 days of implantation. All of these results suggest using bicomponent fibers loaded with DicNa as a valid therapeutic tool capable of supporting the wound healing process and limiting in vivo inflammation and rejection phenomena.
Keywords: animal model; anti-inflammatory drugs; drug delivery; electrospinning; nanofibers.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Optimization of Bicomponent Electrospun Fibers for Therapeutic Use: Post-Treatments to Improve Chemical and Biological Stability.J Funct Biomater. 2017 Oct 16;8(4):47. doi: 10.3390/jfb8040047. J Funct Biomater. 2017. PMID: 29035303 Free PMC article.
-
Controlled release of lawsone from polycaprolactone/gelatin electrospun nano fibers for skin tissue regeneration.Int J Biol Macromol. 2019 Mar 1;124:478-491. doi: 10.1016/j.ijbiomac.2018.11.237. Epub 2018 Nov 27. Int J Biol Macromol. 2019. PMID: 30500508
-
Electrospun Sulfonatocalix[4]arene Loaded Blended Nanofibers: Process Optimization and In Vitro Studies.Pharmaceutics. 2022 Sep 9;14(9):1912. doi: 10.3390/pharmaceutics14091912. Pharmaceutics. 2022. PMID: 36145660 Free PMC article.
-
Novel poly(ε-caprolactone)/gelatin wound dressings prepared by emulsion electrospinning with controlled release capacity of Ketoprofen anti-inflammatory drug.Mater Sci Eng C Mater Biol Appl. 2017 Dec 1;81:459-468. doi: 10.1016/j.msec.2017.08.025. Epub 2017 Aug 10. Mater Sci Eng C Mater Biol Appl. 2017. PMID: 28887998
-
RGD peptide grafted polybutylene adipate-co-terephthalate/gelatin electrospun nanofibers loaded with a matrix metalloproteinase inhibitor drug for alleviating of wounds: an in vitro/in vivo study.Drug Dev Ind Pharm. 2020 Mar;46(3):484-497. doi: 10.1080/03639045.2020.1730397. Epub 2020 Mar 3. Drug Dev Ind Pharm. 2020. PMID: 32077331
Cited by
-
Polycaprolactone for Hard Tissue Regeneration: Scaffold Design and In Vivo Implications.Bioengineering (Basel). 2025 Jan 8;12(1):46. doi: 10.3390/bioengineering12010046. Bioengineering (Basel). 2025. PMID: 39851320 Free PMC article. Review.
References
-
- Lu Y., Li H., Wang J., Yao M., Peng Y., Liu T., Li Z., Luo G., Deng J. Engineering Bacteria-Activated Multifunctionalized Hydrogel for Promoting Diabetic Wound Healing. Adv. Funct. Mater. 2021;31:2105749. doi: 10.1002/adfm.202105749. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

