Uncovering the Therapeutic Potential of Lithium Chloride in Type 2 Diabetic Cardiomyopathy: Targeting Tau Hyperphosphorylation and TGF-β Signaling via GSK-3β Inhibition
- PMID: 39065652
- PMCID: PMC11279906
- DOI: 10.3390/pharmaceutics16070955
Uncovering the Therapeutic Potential of Lithium Chloride in Type 2 Diabetic Cardiomyopathy: Targeting Tau Hyperphosphorylation and TGF-β Signaling via GSK-3β Inhibition
Abstract
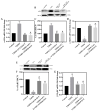
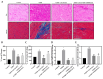
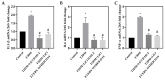
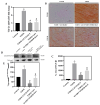
Diabetic cardiomyopathy (DCM) is a major complication of type 2 diabetes mellitus (T2DM) that leads to significant morbidity and mortality. The alteration in the signaling mechanism in diabetes leading to cardiomyopathy remains unclear. The purpose of this study is to investigate the role of tauopathy in myocardial dysfunction observed in T2DM. In that regard, diabetic Sprague Dawley rats were treated with intraperitoneal injections of lithium chloride (LiCl), inhibiting tau phosphorylation. Cardiac function was evaluated, and molecular markers of myocardial fibrosis and the TGF-β signaling were analyzed. T2DM rats exhibited a decline in ejection fraction and fractional shortening that revealed cardiac function abnormalities and increased myocardial fibrosis. These changes were associated with tau hyperphosphorylation. Treating diabetic rats with LiCl attenuated cardiac fibrosis and improved myocardial function. Inhibition of GSK-3β leads to the suppression of tau phosphorylation, which is associated with a decrease in TGF-β expression and regulation of the pro-inflammatory markers, suggesting that tau hyperphosphorylation is parallelly associated with fibrosis and inflammation in the diabetic heart. Our findings provide evidence of a possible role of tau hyperphosphorylation in the pathogenesis of DCM through the activation of TGF-β and by inducing inflammation. Targeting the inhibition of tau phosphorylation may offer novel therapeutic approaches to reduce DCM burden in T2DM patients.
Keywords: TGF-β; diabetic cardiomyopathy; lithium chloride; tau hyperphosphorylation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Alaeddine L.M., Harb F., Hamza M., Dia B., Mogharbil N., Azar N.S., Noureldein M.H., El Khoury M., Sabra R., Eid A.A. Pharmacological regulation of cytochrome P450 metabolites of arachidonic acid attenuates cardiac injury in diabetic rats. Transl. Res. 2021;235:85–101. doi: 10.1016/j.trsl.2021.03.010. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

