A Novel Lactose/MCC/L-HPC Triple-Based Co-Processed Excipients with Improved Tableting Performance Designed for Metoclopramide Orally Disintegrating Tablets
- PMID: 39065656
- PMCID: PMC11279886
- DOI: 10.3390/pharmaceutics16070959
A Novel Lactose/MCC/L-HPC Triple-Based Co-Processed Excipients with Improved Tableting Performance Designed for Metoclopramide Orally Disintegrating Tablets
Abstract
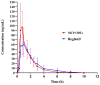
New co-processed excipients comprising lactose (filler and sweetener), microcrystalline cellulose (MCC, filler), and low-substituted hydroxypropyl cellulose (L-HPC, disintegrant and binder) were developed via solvent evaporation for the preparation of metoclopramide orally disintegrating tablets (MCP ODTs). Single-factor and Box-Behnken experimental designs were employed to optimize the formulation. The optimized formulation ratios were water: MCC: lactose (g/g) = 17.26:2.79:4.54:1. The results demonstrated that particles formed by solvent evaporation had superior flowability and compressibility compared to the physical mixture. Tablets compressed with these co-processed excipients exhibited a significantly reduced disintegration time of less than 25 s and achieved complete dissolution within 5 min. Pharmacokinetic studies revealed that MCP ODTs significantly improved Cmax, which was 1.60-fold higher compared to conventional tablets. In summary, the lactose/L-HPC/MCC triple-based co-processed excipients developed in this study are promising and could be successfully utilized in orally disintegrating and fast-release tablets.
Keywords: co-processed excipients; metoclopramide; orally disintegrating tablets; pharmacokinetics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Saharan V.A. Current Advances in Drug Delivery through Fast Dissolving/Disintegrating Dosage Forms. Bentham Science Publishers; Sharjah, United Arab Emirates: 2017.
-
- Al-Zoubi N., Gharaibeh S., Aljaberi A., Nikolakakis I. Spray Drying for Direct Compression of Pharmaceuticals. Processes. 2021;9:267. doi: 10.3390/pr9020267. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

