Examining the Role of Oxytocinergic Signaling and Neuroinflammatory Markers in the Therapeutic Effects of MDMA in a Rat Model for PTSD
- PMID: 39065697
- PMCID: PMC11279644
- DOI: 10.3390/ph17070846
Examining the Role of Oxytocinergic Signaling and Neuroinflammatory Markers in the Therapeutic Effects of MDMA in a Rat Model for PTSD
Abstract
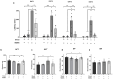
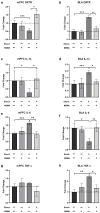
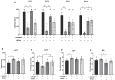
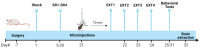
MDMA-assisted psychotherapy has shown potential as an effective treatment for post-traumatic stress disorder (PTSD). Preclinical studies involving rodents have demonstrated that MDMA can facilitate the extinction of fear memories. It has been noted that MDMA impacts oxytocin neurons and pro-inflammatory cytokines. Thus, the aim of this study was to explore the role of oxytocinergic signaling and neuroinflammatory markers in the therapeutic effects of MDMA. To achieve this, male rats were subjected to a model of PTSD involving exposure to shock and situational reminders. MDMA was microinjected into the medial prefrontal cortex (mPFC) before extinction training, followed by behavioral tests assessing activity levels, anxiety, and social function. Our findings indicate that MDMA treatment facilitated fear extinction and mitigated the shock-induced increase in freezing, as well as deficits in social behavior. Shock exposure led to altered expression of the gene coding for OXT-R and neuroinflammation in the mPFC and basolateral amygdala (BLA), which were restored by MDMA treatment. Importantly, the OXT-R antagonist L-368,899 prevented MDMA's therapeutic effects on extinction and freezing behavior. In conclusion, MDMA's therapeutic effects in the PTSD model are associated with alterations in OXT-R expression and neuroinflammation, and MDMA's effects on extinction and anxiety may be mediated by oxytocinergic signaling.
Keywords: MDMA; PTSD; fear extinction; neuroinflammation; oxytocin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Inhibition of serotonin transporters disrupts the enhancement of fear memory extinction by 3,4-methylenedioxymethamphetamine (MDMA).Psychopharmacology (Berl). 2017 Oct;234(19):2883-2895. doi: 10.1007/s00213-017-4684-8. Epub 2017 Jul 24. Psychopharmacology (Berl). 2017. PMID: 28741031 Free PMC article.
-
Effects of Oxytocin on Fear Memory and Neuroinflammation in a Rodent Model of Posttraumatic Stress Disorder.Int J Mol Sci. 2018 Dec 3;19(12):3848. doi: 10.3390/ijms19123848. Int J Mol Sci. 2018. PMID: 30513893 Free PMC article.
-
A proposed mechanism for the MDMA-mediated extinction of traumatic memories in PTSD patients treated with MDMA-assisted therapy.Front Psychiatry. 2022 Oct 12;13:991753. doi: 10.3389/fpsyt.2022.991753. eCollection 2022. Front Psychiatry. 2022. PMID: 36311515 Free PMC article. Review.
-
Effects of oxytocin on prosocial behavior and the associated profiles of oxytocinergic and corticotropin-releasing hormone receptors in a rodent model of posttraumatic stress disorder.J Biomed Sci. 2019 Mar 21;26(1):26. doi: 10.1186/s12929-019-0514-0. J Biomed Sci. 2019. PMID: 30898126 Free PMC article.
-
MDMA-assisted psychotherapy for PTSD: Are memory reconsolidation and fear extinction underlying mechanisms?Prog Neuropsychopharmacol Biol Psychiatry. 2018 Jun 8;84(Pt A):221-228. doi: 10.1016/j.pnpbp.2018.03.003. Epub 2018 Mar 7. Prog Neuropsychopharmacol Biol Psychiatry. 2018. PMID: 29524515 Review.
Cited by
-
How Psychedelics Modulate Multiple Memory Mechanisms in Posttraumatic Stress Disorder.Drugs. 2024 Nov;84(11):1419-1443. doi: 10.1007/s40265-024-02106-4. Epub 2024 Oct 26. Drugs. 2024. PMID: 39455547 Review.
References
-
- Mitchell J.M., Bogenschutz M., Lilienstein A., Harrison C., Kleiman S., Parker-Guilbert K., Ot’alora M.O., Garas W., Paleos C., Gorman I., et al. MDMA-assisted therapy for severe PTSD: A randomized, double-blind, placebo-controlled phase 3 study. Nat. Med. 2021;27:1025–1033. doi: 10.1038/s41591-021-01336-3. - DOI - PMC - PubMed
-
- Mithoefer M.C., Mithoefer A.T., Feduccia A.A., Jerome L., Wagner M., Wymer J., Holland J., Hamilton S., Yazar-Klosinski B., Emerson A., et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: A randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5:486–497. doi: 10.1016/S2215-0366(18)30135-4. - DOI - PubMed
-
- Mithoefer M.C., Wagner M.T., Mithoefer A.T., Jerome L., Doblin R. The safety and efficacy of ±3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: The first randomized controlled pilot study. J. Psychopharmacol. 2011;25:439–452. doi: 10.1177/0269881110378371. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

