Antiproliferative Effect of 7-Ketositosterol in Breast and Liver Cancer Cells: Possible Impact on Ceramide, Extracellular Signal-Regulated Kinases, and Nuclear Factor Kappa B Signaling Pathways
- PMID: 39065711
- PMCID: PMC11279788
- DOI: 10.3390/ph17070860
Antiproliferative Effect of 7-Ketositosterol in Breast and Liver Cancer Cells: Possible Impact on Ceramide, Extracellular Signal-Regulated Kinases, and Nuclear Factor Kappa B Signaling Pathways
Abstract
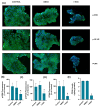
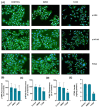
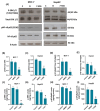
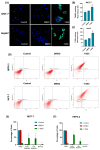
Background: This study aimed to examine the effect of 7-Ketositosterol (7-KSS), on sphingomyelin/ceramide metabolites and apoptosis in human breast MCF-7 and human liver HepG2 cancer cells. Methods: Anti-proliferative effects of 7-KSS treatment were assessed at different concentrations and periods. Cell viability was assessed through MTT analysis, whereas the levels of sphingosine-1-phosphate (S1P), sphingomyelins (SMs), and ceramides (CERs) were measured using LC-MS/MS. Phosphorylated 44/42 ERK1/2 and NF-κB p65 (Ser536) protein levels were measured by Western blot analysis and immunofluorescence staining. Apoptosis was evaluated by TUNEL staining and flow cytometric assessment of annexin-V and propidium iodide (PI) labeling. Results: Treatment with 7-KSS significantly decreased cell survival and S1P, p-44/42 ERK1/2, and p-NF-κB p65 protein levels in cancer cells compared to controls. A substantial rise was detected in intracellular amounts of C16-C24 CERs and apoptosis in cancer cells incubated with 7-KSS. Conclusions: 7-KSS stimulated ceramide accumulation and apoptosis while decreasing cell proliferation via downregulating S1P, p-44/42 ERK1/2, and p-NF-κB p65 protein levels.
Keywords: 7-Ketositosterol; ERK; apoptosis; ceramide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Antiproliferative Effects of Thymoquinone in MCF-7 Breast and HepG2 Liver Cancer Cells: Possible Role of Ceramide and ER Stress.Nutr Cancer. 2021;73(3):460-472. doi: 10.1080/01635581.2020.1751216. Epub 2020 Apr 14. Nutr Cancer. 2021. PMID: 32286088
-
Sparstolonin B Reduces Estrogen-Dependent Proliferation in Cancer Cells: Possible Role of Ceramide and PI3K/AKT/mTOR Inhibition.Pharmaceuticals (Basel). 2024 Nov 21;17(12):1564. doi: 10.3390/ph17121564. Pharmaceuticals (Basel). 2024. PMID: 39770406 Free PMC article.
-
Effect of ER stress on sphingolipid levels and apoptotic pathways in retinal pigment epithelial cells.Redox Biol. 2020 Feb;30:101430. doi: 10.1016/j.redox.2020.101430. Epub 2020 Jan 20. Redox Biol. 2020. PMID: 31978676 Free PMC article.
-
A Rheostat of Ceramide and Sphingosine-1-Phosphate as a Determinant of Oxidative Stress-Mediated Kidney Injury.Int J Mol Sci. 2022 Apr 4;23(7):4010. doi: 10.3390/ijms23074010. Int J Mol Sci. 2022. PMID: 35409370 Free PMC article. Review.
-
The Role of Sphingosine-1-Phosphate and Ceramide-1-Phosphate in Inflammation and Cancer.Mediators Inflamm. 2017;2017:4806541. doi: 10.1155/2017/4806541. Epub 2017 Nov 15. Mediators Inflamm. 2017. PMID: 29269995 Free PMC article. Review.
Cited by
-
From laboratory to clinic: opportunities and challenges of functional food active ingredients in cancer therapy.Front Nutr. 2025 Jul 30;12:1627949. doi: 10.3389/fnut.2025.1627949. eCollection 2025. Front Nutr. 2025. PMID: 40808836 Free PMC article. Review.
References
-
- El Omari N., Bakrim S., Khalid A., Abdalla A.N., Iesa M.A.M., El Kadri K., Tang S.Y., Goh B.H., Bouyahya A. Unveiling the molecular mechanisms: Dietary phytosterols as guardians against cardiovascular diseases. Nat. Prod. Bioprospect. 2024;14:27. doi: 10.1007/s13659-024-00451-1. - DOI - PMC - PubMed
-
- Khan Z., Nath N., Rauf A., Bin Emran T., Mitra S., Islam F., Chandran D., Barua J., Khandaker M.U., Idris A.M., et al. Multifunctional roles and pharmacological potential of β-sitosterol: Emerging evidence toward clinical applications. Chem. Interact. 2022;365:110117. doi: 10.1016/j.cbi.2022.110117. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

