Oral Treatment with the Pectin Fibre Obtained from Yellow Passion Fruit Peels Worsens Sepsis Outcome in Mice by Affecting the Intestinal Barrier
- PMID: 39065714
- PMCID: PMC11279511
- DOI: 10.3390/ph17070863
Oral Treatment with the Pectin Fibre Obtained from Yellow Passion Fruit Peels Worsens Sepsis Outcome in Mice by Affecting the Intestinal Barrier
Abstract
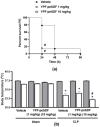
The biological activities of plant-derived soluble dietary fibres (SDFs) have been widely investigated. Pectin from yellow passion fruit (YPF-peSDF) peels was suggested as a protective macromolecule in ulcers and colitis due to its antioxidant and anti-inflammatory properties. Sepsis has high mortality and morbidity and is characterised by inflammatory and oxidative stress imbalances. Evidence suggests that pectins may aid sepsis treatment; however, the effects of YPF-peSDF on sepsis remain unclear. Herein, polymicrobial sepsis was induced by cecal-ligation and puncture in mice treated with YPF-peSDF (1 and 10 mg/kg; gavage). YPF-peSDF accelerated mortality, reaching 100% in 24 h. Inflammation was present in the colons and small intestines (SI) of both vehicle- and fibre-treated mice. Although crypt depth and width, and villus height were preserved in the SI of septic mice administered YPF-peSDF, they exhibited exacerbated muscle layer atrophy and mucosa and submucosa hypertrophy, along with shortened enterocytes. Larger crypts and shorter enterocytes were noted in their colons in comparison with vehicle-controls. YPF-peSDF also reduced inflammatory cell numbers and exacerbated IL-6 levels in peritoneal lavage fluid (PELF) samples. YPF-peSDF modulated SI but not colon cytokines. Lipoperoxidation and antioxidant capacity levels were attenuated in PELF samples. Overall, in contrast to previous evidence, YPF-peSDF worsened polymicrobial sepsis outcomes in mice.
Keywords: pectin; sepsis; soluble dietary fibres.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Development and characterization of an aromatic and antioxidant active film based on myofibrillar protein from fish by-products and passion fruit essential oil.Food Chem. 2025 May 15;474:143125. doi: 10.1016/j.foodchem.2025.143125. Epub 2025 Jan 28. Food Chem. 2025. PMID: 39893724
-
Inhibition of IKKβ in enterocytes exacerbates sepsis-induced intestinal injury and worsens mortality.Crit Care Med. 2013 Oct;41(10):e275-85. doi: 10.1097/CCM.0b013e31828a44ed. Crit Care Med. 2013. PMID: 23939348 Free PMC article.
-
Epidermal Growth Factor Improves Intestinal Integrity and Survival in Murine Sepsis Following Chronic Alcohol Ingestion.Shock. 2017 Feb;47(2):184-192. doi: 10.1097/SHK.0000000000000709. Shock. 2017. PMID: 27465753 Free PMC article.
-
Fecal Microbiota Transplantation and Hydrocortisone Ameliorate Intestinal Barrier Dysfunction and Improve Survival in a Rat Model of Cecal Ligation and Puncture-Induced Sepsis.Shock. 2021 May 1;55(5):666-675. doi: 10.1097/SHK.0000000000001566. Shock. 2021. PMID: 32496421
-
Baicalin improves survival in a murine model of polymicrobial sepsis via suppressing inflammatory response and lymphocyte apoptosis.PLoS One. 2012;7(5):e35523. doi: 10.1371/journal.pone.0035523. Epub 2012 May 8. PLoS One. 2012. PMID: 22590504 Free PMC article.
References
-
- da Silva K.S., Abboud K.Y., Schiebel C.S., de Oliveira N.M.T., Bueno L.R., de Mello Braga L.L.V., da Silveira B.C., Santos I.W.F.d., Gomes E.d.S., Gois M.B., et al. Polysaccharides from Passion Fruit Peels: From an Agroindustrial By-Product to a Viable Option for 5-FU-Induced Intestinal Damage. Pharmaceuticals. 2023;16:912. doi: 10.3390/ph16070912. - DOI - PMC - PubMed
-
- Qiao H., Zhao T., Yin J., Zhang Y., Ran H., Chen S., Wu Z., Zhang R., Wang X., Gan L., et al. Structural Characteristics of Inulin and Microcrystalline Cellulose and Their Effect on Ameliorating Colitis and Altering Colonic Microbiota in Dextran Sodium Sulfate-Induced Colitic Mice. ACS Omega. 2022;7:10921–10932. doi: 10.1021/acsomega.1c06552. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

