Enhanced Antitumor Activity by the Combination of Dasatinib and Selinexor in Chronic Myeloid Leukemia
- PMID: 39065744
- PMCID: PMC11279392
- DOI: 10.3390/ph17070894
Enhanced Antitumor Activity by the Combination of Dasatinib and Selinexor in Chronic Myeloid Leukemia
Abstract
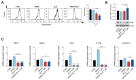
Background: Chronic myeloid leukemia is a hematological malignancy characterized by the abnormal proliferation of leukemic cells. Despite significant progress with tyrosine kinase inhibitors, such as Dasatinib, resistance remains a challenge. The aim of the present study was to investigate the potential of Selinexor, an Exportin-1 inhibitor, to improve TKI effectiveness on CML.
Methods: Human CML cell lines (LAMA84 and K562) were treated with Selinexor, Dasatinib, or their combination. Apoptosis, mitochondrial membrane potential, and mitochondrial mass were assessed using flow cytometry. Real-time RT-PCR was used to evaluate the expression of genes related to mitochondrial function. Western blot and confocal microscopy examined PINK and heme oxygenase-1 (HO-1) protein levels.
Results: Selinexor induced apoptosis and mitochondrial depolarization in CML cell lines, reducing cell viability. The Dasatinib/Selinexor combination further enhanced cytotoxicity, modified mitochondrial fitness, and downregulated HO-1 nuclear translocation, which has been associated with drug resistance in different models.
Conclusions: In conclusion, this study suggests that Dasatinib/Selinexor could be a promising therapeutic strategy for CML, providing new insights for new targeted therapies.
Keywords: Selinexor; chronic myeloid leukemia; mitochondria; tyrosine kinase inhibitors.
Conflict of interest statement
The author declares no conflicts of interest.
Figures



Similar articles
-
Dynamic Single-Cell RNA-Seq reveals mechanism of Selinexor-Resistance in Chronic myeloid leukemia.Int Immunopharmacol. 2024 Jun 15;134:112212. doi: 10.1016/j.intimp.2024.112212. Epub 2024 May 9. Int Immunopharmacol. 2024. PMID: 38728882
-
Expression of LYN and PTEN genes in chronic myeloid leukemia and their importance in therapeutic strategy.Blood Cells Mol Dis. 2014 Feb-Mar;52(2-3):121-5. doi: 10.1016/j.bcmd.2013.09.002. Epub 2013 Oct 3. Blood Cells Mol Dis. 2014. PMID: 24091144
-
KPT-330 inhibition of chromosome region maintenance 1 is cytotoxic and sensitizes chronic myeloid leukemia to Imatinib.Cell Death Discov. 2018 Apr 23;4:48. doi: 10.1038/s41420-018-0049-2. eCollection 2018. Cell Death Discov. 2018. PMID: 29707241 Free PMC article.
-
Characterization of cancer stem cells in chronic myeloid leukaemia.Biochem Soc Trans. 2007 Nov;35(Pt 5):1347-51. doi: 10.1042/BST0351347. Biochem Soc Trans. 2007. PMID: 17956348 Review.
-
Selinexor in acute myeloid leukemia: therapeutic applications and current challenges.Front Pharmacol. 2025 May 20;16:1602911. doi: 10.3389/fphar.2025.1602911. eCollection 2025. Front Pharmacol. 2025. PMID: 40463909 Free PMC article. Review.
Cited by
-
Advances and Challenges in Targeted Therapy and Its Combination Strategies for Leukemia.Biomedicines. 2025 Jul 7;13(7):1652. doi: 10.3390/biomedicines13071652. Biomedicines. 2025. PMID: 40722724 Free PMC article. Review.
References
-
- Giallongo C., Parrinello N.L., La Cava P., Camiolo G., Romano A., Scalia M., Stagno F., Palumbo G.A., Avola R., Li Volti G., et al. Monocytic myeloid-derived suppressor cells as prognostic factor in chronic myeloid leukaemia patients treated with dasatinib. J. Cell Mol. Med. 2018;22:1070–1080. doi: 10.1111/jcmm.13326. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

