Pirfenidone in Idiopathic Pulmonary Fibrosis: Real-World Observation on Efficacy and Safety, Focus on Patients Undergoing Antithrombotic and Anticoagulant
- PMID: 39065780
- PMCID: PMC11280355
- DOI: 10.3390/ph17070930
Pirfenidone in Idiopathic Pulmonary Fibrosis: Real-World Observation on Efficacy and Safety, Focus on Patients Undergoing Antithrombotic and Anticoagulant
Abstract
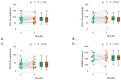
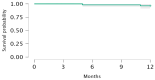
Idiopathic pulmonary fibrosis (IPF) is a rare and progressive interstitial lung disease characterized by irreversible distortion of lung architecture and subsequent loss of pulmonary function. Pirfenidone is an antifibrotic agent associated with increased progression-free survival and overall survival rates, but it carries multiple side effects. The aim of the study was to examine the efficacy and safety profile of pirfenidone in a real-life context, with a focus on the concomitant use of antithrombotic and/or anticoagulant treatments. The clinical and functional data (forced vital capacity [FVC], forced expiratory volume in 1 s [FEV1], diffusing lung capacity for carbon monoxide [DLCO], and 6 min walking test distance [6MWD]) of all IPF patients treated with pirfenidone and referred to our two centers between 2019 and 2022 were retrospectively analyzed at baseline, 6 and 12 months after the start of treatment. A total of 55 IPF subjects undergoing pirfenidone treatment were included in the analysis (45.5% females, median [IQR] age at disease onset 68.0 [10.0] years, median [IQR] age at baseline 69.0 [10.8] years). Compared to baseline, at 12 months, FVC (86.0% vs. 80.0%; p = 0.023) and DLCO (44.0% vs. 40.0%; p = 0.002) were significantly reduced, while FEV1 (p = 0.304) and 6MWD (p = 0.276) remained stable; no significant change was recorded at 6 months. Most of the reported adverse events were mild or moderate. Gastrointestinal intolerance (9.1%) was the main cause of treatment discontinuation. A total of 5% of patients reported at least one minor bleeding event, although all episodes occurred in those receiving concomitant antithrombotic or anticoagulant. Overall, this real-life experience confirms the efficacy and safety profile of pirfenidone in the case of the concomitant use of antithrombotic and/or anticoagulant drugs.
Keywords: anticoagulants; antithrombotic treatment; idiopathic pulmonary fibrosis (IPF); interstitial lung disease (ILD); pirfenidone; pulmonary function test (PFT).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Real-life experiences in a single center: efficacy of pirfenidone in idiopathic pulmonary fibrosis and fibrotic idiopathic non-specific interstitial pneumonia patients.Ther Adv Respir Dis. 2020 Jan-Dec;14:1753466620963015. doi: 10.1177/1753466620963015. Ther Adv Respir Dis. 2020. PMID: 33070705 Free PMC article.
-
Real-world insights into safety, tolerability, and predictive factors of adverse drug reactions in treating idiopathic pulmonary fibrosis with pirfenidone and nintedanib.Ther Adv Drug Saf. 2025 May 27;16:20420986251341645. doi: 10.1177/20420986251341645. eCollection 2025. Ther Adv Drug Saf. 2025. PMID: 40438276 Free PMC article.
-
Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial.Lancet Respir Med. 2020 Feb;8(2):147-157. doi: 10.1016/S2213-2600(19)30341-8. Epub 2019 Sep 29. Lancet Respir Med. 2020. PMID: 31578169 Clinical Trial.
-
Role of pirfenidone in the management of pulmonary fibrosis.Ther Clin Risk Manag. 2017 Apr 3;13:427-437. doi: 10.2147/TCRM.S81141. eCollection 2017. Ther Clin Risk Manag. 2017. PMID: 28435277 Free PMC article. Review.
-
The efficacy and safety of pirfenidone in the treatment of HPS-related pulmonary fibrosis and Idiopathic pulmonary fibrosis: a systematic review and meta-analysis.Eur Rev Med Pharmacol Sci. 2022 Nov;26(22):8411-8424. doi: 10.26355/eurrev_202211_30377. Eur Rev Med Pharmacol Sci. 2022. PMID: 36459024
Cited by
-
Real-World Experience in the Clinical Use of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis in Taiwan: A Post-Marketing Surveillance Study.Biomedicines. 2024 Oct 15;12(10):2348. doi: 10.3390/biomedicines12102348. Biomedicines. 2024. PMID: 39457660 Free PMC article.
-
The Impact of Comorbidities on the Discontinuation of Antifibrotic Therapy in Patients with Idiopathic Pulmonary Fibrosis.Pharmaceuticals (Basel). 2025 Mar 14;18(3):411. doi: 10.3390/ph18030411. Pharmaceuticals (Basel). 2025. PMID: 40143187 Free PMC article.
-
Epithelial Cell Dysfunction in Pulmonary Fibrosis: Mechanisms, Interactions, and Emerging Therapeutic Targets.Pharmaceuticals (Basel). 2025 May 28;18(6):812. doi: 10.3390/ph18060812. Pharmaceuticals (Basel). 2025. PMID: 40573209 Free PMC article. Review.
-
Timing impact on the initiation of pirfenidone therapy on idiopathic pulmonary fibrosis disease progression.World J Clin Cases. 2024 Nov 16;12(32):6538-6542. doi: 10.12998/wjcc.v12.i32.6538. World J Clin Cases. 2024. PMID: 39554893 Free PMC article.
-
Comorbidities' Effect on IPF: Pathogenesis and Management.Biomedicines. 2025 Jun 1;13(6):1362. doi: 10.3390/biomedicines13061362. Biomedicines. 2025. PMID: 40564081 Free PMC article. Review.
References
-
- Gahl W.A., Brantly M., Kaiser-Kupfer M.I., Iwata F., Hazelwood S., Shotelersuk V., Duffy L.F., Kuehl E.M., Troendle J., Bernardini I. Genetic defects and clinical characteristics of patients with a form of oculocutaneous albinism (Hermansky-Pudlak syndrome) N. Engl. J. Med. 1998;338:1258–1264. doi: 10.1056/NEJM199804303381803. - DOI - PubMed
LinkOut - more resources
Full Text Sources

