Development of Dry and Liquid Duplex Reagent Mix-Based Polymerase Chain Reaction Assays as Novel Tools for the Rapid and Easy Quantification of Bovine Leukemia Virus (BLV) Proviral Loads
- PMID: 39066179
- PMCID: PMC11281531
- DOI: 10.3390/v16071016
Development of Dry and Liquid Duplex Reagent Mix-Based Polymerase Chain Reaction Assays as Novel Tools for the Rapid and Easy Quantification of Bovine Leukemia Virus (BLV) Proviral Loads
Abstract
Bovine leukemia virus (BLV) is prevalent worldwide, causing serious problems in the cattle industry. The BLV proviral load (PVL) is a useful index for estimating disease progression and transmission risk. We previously developed a quantitative real-time PCR (qPCR) assay to measure the PVL using the coordination of common motif (CoCoMo) degenerate primers. Here, we constructed a novel duplex BLV-CoCoMo qPCR assay that can amplify two genes simultaneously using a FAM-labeled MGB probe for the BLV LTR gene and a VIC-labeled MGB probe for the BoLA-DRA gene. This liquid duplex assay maintained its original sensitivity and reproducibility in field samples. Furthermore, we developed a dry duplex assay composed of PCR reagents necessary for the optimized liquid duplex assay. We observed a strong positive correlation between the PVLs measured using the dry and liquid duplex assays. Validation analyses showed that the sensitivity of the dry duplex assay was slightly lower than that of the other methods for the detection of a BLV molecular clone, but it showed similar sensitivity to the singleplex assay and slightly higher sensitivity than the liquid duplex assay for the PVL quantification of 82 field samples. Thus, our liquid and dry duplex assays are useful for measuring the BLV PVL in field samples, similar to the original singleplex assay.
Keywords: bovine leukemia virus (BLV); dry; duplex; liquid; proviral load (PVL); quantitative real-time PCR (qPCR); singleplex.
Conflict of interest statement
K.S. (Kazuyuki Shoji), M.I. and M.O. are employed by Nippon Gene Co., Ltd. The remaining co-authors declare that they have no competing interests.
Figures

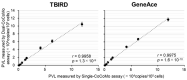

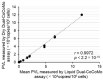
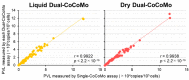

References
-
- EFSA AHAW Panel on Animal Health and Welfare Scientific opinion on enzootic bovine leukosis. EFSA J. 2015;13:4188.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

