Elevated Inflammation Associated with Markers of Neutrophil Function and Gastrointestinal Disruption in Pilot Study of Plasmodium fragile Co-Infection of ART-Treated SIVmac239+ Rhesus Macaques
- PMID: 39066199
- PMCID: PMC11281461
- DOI: 10.3390/v16071036
Elevated Inflammation Associated with Markers of Neutrophil Function and Gastrointestinal Disruption in Pilot Study of Plasmodium fragile Co-Infection of ART-Treated SIVmac239+ Rhesus Macaques
Abstract
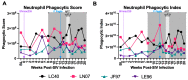
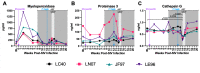
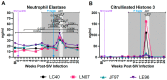
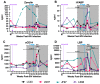
Human immunodeficiency virus (HIV) and malaria, caused by infection with Plasmodium spp., are endemic in similar geographical locations. As a result, there is high potential for HIV/Plasmodium co-infection, which increases the pathology of both diseases. However, the immunological mechanisms underlying the exacerbated disease pathology observed in co-infected individuals are poorly understood. Moreover, there is limited data available on the impact of Plasmodium co-infection on antiretroviral (ART)-treated HIV infection. Here, we used the rhesus macaque (RM) model to conduct a pilot study to establish a model of Plasmodium fragile co-infection during ART-treated simian immunodeficiency virus (SIV) infection, and to begin to characterize the immunopathogenic effect of co-infection in the context of ART. We observed that P. fragile co-infection resulted in parasitemia and anemia, as well as persistently detectable viral loads (VLs) and decreased absolute CD4+ T-cell counts despite daily ART treatment. Notably, P. fragile co-infection was associated with increased levels of inflammatory cytokines, including monocyte chemoattractant protein 1 (MCP-1). P. fragile co-infection was also associated with increased levels of neutrophil elastase, a plasma marker of neutrophil extracellular trap (NET) formation, but significant decreases in markers of neutrophil degranulation, potentially indicating a shift in the neutrophil functionality during co-infection. Finally, we characterized the levels of plasma markers of gastrointestinal (GI) barrier permeability and microbial translocation and observed significant correlations between indicators of GI dysfunction, clinical markers of SIV and Plasmodium infection, and neutrophil frequency and function. Taken together, these pilot data verify the utility of using the RM model to examine ART-treated SIV/P. fragile co-infection, and indicate that neutrophil-driven inflammation and GI dysfunction may underlie heightened SIV/P. fragile co-infection pathogenesis.
Keywords: Plasmodium fragile; co-infection; immunology; malaria; neutrophils; nonhuman primate; simian immunodeficiency virus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
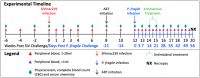
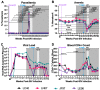
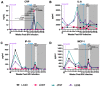
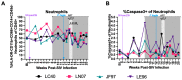
References
-
- WHO . HIV Data and Statistics 2022. WHO; Geneva, Switzerland: 2022. [(accessed on 11 May 2023)]. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/s....
-
- WHO . World Malaria Report 2023. WHO; Geneva, Switzerland: 2023. [(accessed on 12 December 2023)]. Available online: https://www.who.int/publications/i/item/9789240086173.
-
- Finzi D., Blankson J., Siliciano J.D., Margolick J.B., Chadwick K., Pierson T., Smith K., Lisziewicz J., Lori F., Flexner C., et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 1999;5:512–517. doi: 10.1038/8394. - DOI - PubMed
-
- Holkmann Olsen C., Mocroft A., Kirk O., Vella S., Blaxhult A., Clumeck N., Fisher M., Katlama C., Phillips A.N., Lundgren J.D. Interruption of combination antiretroviral therapy and risk of clinical disease progression to AIDS or death. HIV Med. 2007;8:96–104. doi: 10.1111/j.1468-1293.2007.00436.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

