Saliva Is a Sensitive and Accessible Sample Both for SARS-CoV-2 Detection and for the Evaluation of Treatment Effectiveness in Follow-Up Studies
- PMID: 39066203
- PMCID: PMC11281700
- DOI: 10.3390/v16071040
Saliva Is a Sensitive and Accessible Sample Both for SARS-CoV-2 Detection and for the Evaluation of Treatment Effectiveness in Follow-Up Studies
Abstract
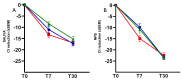
Despite emerging evidence indicating that molecular SARS-CoV-2 tests performed on saliva have diagnostic sensitivity and specificity comparable to those observed with nasopharyngeal swabs (NPSs), most in vivo follow-up studies on the efficacy of drugs against SARS-CoV-2 have been performed on NPSs, not considering saliva as a possible alternative matrix. For this reason, in this study, we used, in parallel, saliva and NPS samples for the detection of SARS-CoV-2 by real-time RT-PCR in patients receiving Tixagevimab/Cilgavimab, Nirmatrelvir/Ritonavir, or Sotrovimab as a treatment against SARS-CoV-2. Our results showed a good correlation between the NPS and saliva samples for each drug; moreover, comparable changes in the cycle threshold (Ct) levels in saliva and NPSs were observed both 7 days and 30 days after treatment, thus confirming that the saliva represents a good matrix for in vivo follow-up studies verifying the effectiveness of treatments against SARS-CoV-2.
Keywords: COVID-19 diagnosis; SARS-CoV-2; antiviral therapy; follow-up studies; molecular assay; monoclonal antibodies; saliva.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- To K.K.W., Yip C.C.Y., Lai C.Y.W., Wong C.K.H., Ho D.T.Y., Pang P.K.P., Ng A.C.K., Leung K.H., Poon R.W.S., Chan K.H., et al. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: A diagnostic validity study. Clin. Microbiol. Infect. 2019;25:372–378. doi: 10.1016/j.cmi.2018.06.009. - DOI - PubMed
-
- Butler-Laporte G., Lawandi A., Schiller I., Yao M., Dendukuri N., McDonald E.G., Lee T.C. Comparison of Saliva and Nasopharyngeal Swab Nucleic Acid Amplification Testing for Detection of SARS-CoV-2: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2021;181:353–360. doi: 10.1001/jamainternmed.2020.8876. - DOI - PMC - PubMed
-
- Tobik E.R., Kitfield-Vernon L.B., Thomas R.J., Steel S.A., Tan S.H., Allicock O.M., Choate B.L., Akbarzada S., Wyllie A.L. Saliva as a sample type for SARS-CoV-2 detection: Implementation successes and opportunities around the globe. Expert Rev. Mol. Diagn. 2022;22:519–535. doi: 10.1080/14737159.2022.2094250. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

