Short Treatment of 42 Days with Oral GS-441524 Results in Equal Efficacy as the Recommended 84-Day Treatment in Cats Suffering from Feline Infectious Peritonitis with Effusion-A Prospective Randomized Controlled Study
- PMID: 39066306
- PMCID: PMC11281457
- DOI: 10.3390/v16071144
Short Treatment of 42 Days with Oral GS-441524 Results in Equal Efficacy as the Recommended 84-Day Treatment in Cats Suffering from Feline Infectious Peritonitis with Effusion-A Prospective Randomized Controlled Study
Abstract
In the past, feline infectious peritonitis (FIP) caused by feline coronavirus (FCoV) was considered fatal. Today, highly efficient drugs, such as GS-441524, can lead to complete remission. The currently recommended treatment duration in the veterinary literature is 84 days. This prospective randomized controlled treatment study aimed to evaluate whether a shorter treatment duration of 42 days with oral GS-441524 obtained from a licensed pharmacy is equally effective compared to the 84-day regimen. Forty cats with FIP with effusion were prospectively included and randomized to receive 15 mg/kg of GS-441524 orally every 24h (q24h), for either 42 or 84 days. Cats were followed for 168 days after treatment initiation. With the exception of two cats that died during the treatment, 38 cats (19 in short, 19 in long treatment group) recovered with rapid improvement of clinical and laboratory parameters as well as a remarkable reduction in viral loads in blood and effusion. Orally administered GS-441524 given as a short treatment was highly effective in curing FIP without causing serious adverse effects. All cats that completed the short treatment course successfully were still in complete remission on day 168. Therefore, a shorter treatment duration of 42 days GS-441524 15 mg/kg can be considered equally effective.
Keywords: BOVA; FCoV; FIP; GS-441524; antiviral chemotherapy; feline coronavirus; therapy; treatment duration.
Conflict of interest statement
The authors declare that they have no conflict of interest. The GS-441524 tablets were provided by BOVA Specials, London, UK, but BOVA played no role in the interpretation of study data or the decision to submit the manuscript for publication. No commercial conflict of interest exists as the information is solely for scientific dissemination.
Figures


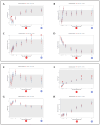
 : start of treatment with GS-441524;
: start of treatment with GS-441524;  : end of treatment of 20/40 cats on day 42;
: end of treatment of 20/40 cats on day 42;  : end of treatment of 20/40 cats on day 84;
: end of treatment of 20/40 cats on day 84;  : evaluation of laboratory parameters;
: evaluation of laboratory parameters;  : measurement of virology parameters (including viral loads in blood, effusion and feces, anti-FCoV antibodies);
: measurement of virology parameters (including viral loads in blood, effusion and feces, anti-FCoV antibodies);  : abdominal ultrasonography;
: abdominal ultrasonography;  : cardiologic examination;
: cardiologic examination;  : neurologic examination.
: neurologic examination.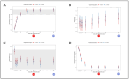






References
-
- Pedersen N.C. Virologic and immunologic aspects of feline infectious peritonitis virus infection. In: Lai M.M.C., Stohlman S.A., editors. Coronaviruses. Springer; Boston, MA, USA: 1987. pp. 529–550. - PubMed
-
- Murphy B.G., Perron M., Murakami E., Bauer K., Park Y., Eckstrand C., Liepnieks M., Pedersen N.C. The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies. Vet. Microbiol. 2018;219:226–233. doi: 10.1016/j.vetmic.2018.04.026. - DOI - PMC - PubMed
-
- Pedersen N.C., Perron M., Bannasch M., Montgomery E., Murakami E., Liepnieks M., Liu H. Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis. J. Feline Med. Surg. 2019;21:271–281. doi: 10.1177/1098612X19825701. - DOI - PMC - PubMed
-
- Dickinson P.J., Bannasch M., Thomasy S.M., Murthy V.D., Vernau K.M., Liepnieks M., Montgomery E., Knickelbein K.E., Murphy B., Pedersen N.C. Antiviral treatment using the adenosine nucleoside analogue GS-441524 in cats with clinically diagnosed neurological feline infectious peritonitis. J. Vet. Intern. Med. 2020;34:1587–1593. doi: 10.1111/jvim.15780. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

