HIV Persistence, Latency, and Cure Approaches: Where Are We Now?
- PMID: 39066325
- PMCID: PMC11281696
- DOI: 10.3390/v16071163
HIV Persistence, Latency, and Cure Approaches: Where Are We Now?
Abstract
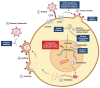
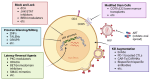
The latent reservoir remains a major roadblock to curing human immunodeficiency virus (HIV) infection. Currently available antiretroviral therapy (ART) can suppress active HIV replication, reduce viral loads to undetectable levels, and halt disease progression. However, antiretroviral drugs are unable to target cells that are latently infected with HIV, which can seed viral rebound if ART is stopped. Consequently, a major focus of the field is to study the latent viral reservoir and develop safe and effective methods to eliminate it. Here, we provide an overview of the major mechanisms governing the establishment and maintenance of HIV latency, the key challenges posed by latent reservoirs, small animal models utilized to study HIV latency, and contemporary cure approaches. We also discuss ongoing efforts to apply these approaches in combination, with the goal of achieving a safe, effective, and scalable cure for HIV that can be extended to the tens of millions of people with HIV worldwide.
Keywords: HIV; animal model; cure; latency; persistence; reservoir.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- HIV/AIDS J.U.N.P. UNAIDS Global AIDS/HIV Statistics Fact Sheet. UNAIDS; Geneva, Switzerland: 2023. Joint United Nations Programme on HIV/AIDS 2023.
-
- Department of Health and Human Services . Panel on Antiretroviral Guidelines for Adults and Adolescents. Department of Health and Human Services; Washington, DC, USA: 2024. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV.
-
- Finzi D., Hermankova M., Pierson T., Carruth L.M., Buck C., Chaisson R.E., Quinn T.C., Chadwick K., Margolick J., Brookmeyer R., et al. Identification of a Reservoir for HIV-1 in Patients on Highly Active Antiretroviral Therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. - DOI - PubMed
-
- Finzi D., Blankson J., Siliciano J.D., Margolick J.B., Chadwick K., Pierson T., Smith K., Lisziewicz J., Lori F., Flexner C., et al. Latent Infection of CD4+ T Cells Provides a Mechanism for Lifelong Persistence of HIV-1, Even in Patients on Effective Combination Therapy. Nat. Med. 1999;5:512–517. doi: 10.1038/8394. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

