Feline Infectious Peritonitis mRNA Vaccine Elicits Both Humoral and Cellular Immune Responses in Mice
- PMID: 39066343
- PMCID: PMC11281389
- DOI: 10.3390/vaccines12070705
Feline Infectious Peritonitis mRNA Vaccine Elicits Both Humoral and Cellular Immune Responses in Mice
Abstract
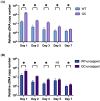
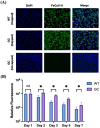
Feline infectious peritonitis (FIP) is a devastating and often fatal disease caused by feline coronavirus (FCoV). Currently, there is no widely used vaccine for FIP, and many attempts using a variety of platforms have been largely unsuccessful due to the disease's highly complicated pathogenesis. One such complication is antibody-dependent enhancement (ADE) seen in FIP, which occurs when sub-neutralizing antibody responses to viral surface proteins paradoxically enhance disease. A novel vaccine strategy is presented here that can overcome the risk of ADE by instead using a lipid nanoparticle-encapsulated mRNA encoding the transcript for the internal structural nucleocapsid (N) FCoV protein. Both wild type and, by introduction of silent mutations, GC content-optimized mRNA vaccines targeting N were developed. mRNA durability in vitro was characterized by quantitative reverse-transcriptase PCR and protein expression by immunofluorescence assay for one week after transfection of cultured feline cells. Both mRNA durability and protein production in vitro were improved with the GC-optimized construct as compared to wild type. Immune responses were assayed by looking at N-specific humoral (by ELISA) and stimulated cytotoxic T cell (by flow cytometry) responses in a proof-of-concept mouse vaccination study. These data together demonstrate that an LNP-mRNA FIP vaccine targeting FCoV N is stable in vitro, capable of eliciting an immune response in mice, and provides justification for beginning safety and efficacy trials in cats.
Keywords: feline coronavirus; feline infectious peritonitis; mRNA vaccine; nucleocapsid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- MacLachlan N., Dubovi E. Coronaviridae. Academic Press; Burlington, MA, USA: 2017.
-
- Tuanthap S., Chiteafea N., Rattanasrisomporn J., Choowongkomon K. Comparative Sequence Analysis of the Accessory and Nucleocapsid Genes of Feline Coronavirus Strains Isolated from Cats Diagnosed with Effusive Feline Infectious Peritonitis. Arch. Virol. 2021;166:2779–2787. doi: 10.1007/S00705-021-05188-7. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

