Deciphering the Potential of Probiotics in Vaccines
- PMID: 39066349
- PMCID: PMC11281421
- DOI: 10.3390/vaccines12070711
Deciphering the Potential of Probiotics in Vaccines
Abstract
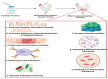
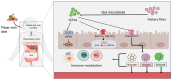
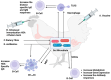
The demand for vaccines, particularly those prepared from non-conventional sources, is rising due to the emergence of drug resistance around the globe. Probiotic-based vaccines are a wise example of such vaccines which represent new horizons in the field of vaccinology in providing an enhanced and diversified immune response. The justification for incorporating probiotics into vaccines lies in the fact that that they hold the capacity to regulate immune function directly or indirectly by influencing the gastrointestinal microbiota and related pathways. Several animal-model-based studies have also highlighted the efficacy of these vaccines. The aim of this review is to collect and summarize the trends in the recent scientific literature regarding the role of probiotics in vaccines and vaccinology, along with their impact on target populations.
Keywords: efficacy; immune response; probiotics; safety; vaccine development.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- FAO. WHO . Cordoba, Argentina: Food and Agriculture Organization of the United Nations and World Health Organization Expert Consultation Report. FAO; Rome, Italy: WHO; Geneva, Switzerland: 2001. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria.
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

