Long-Lasting Enhanced Cytokine Responses Following SARS-CoV-2 BNT162b2 mRNA Vaccination
- PMID: 39066374
- PMCID: PMC11281652
- DOI: 10.3390/vaccines12070736
Long-Lasting Enhanced Cytokine Responses Following SARS-CoV-2 BNT162b2 mRNA Vaccination
Abstract
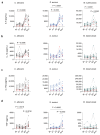
The mRNA vaccine against COVID-19 protects against severe disease by the induction of robust humoral and cellular responses. Recent studies have shown the capacity of some vaccines to induce enduring non-specific innate immune responses by the induction of trained immunity, augmenting protection against unrelated pathogens. This study aimed to assess whether the mRNA vaccine BNT162b2 can induce lasting non-specific immune responses in myeloid cells following a three-dose vaccination scheme. In a sample size consisting of 20 healthy individuals from Romania, we assessed inflammatory proteins using the Olink® Target 96 Inflammation panel, as well as ex vivo cytokine responses following stimulations with unrelated PRR ligands. We assessed the vaccine-induced non-specific systemic inflammation and functional adaptations of myeloid cells. Our results revealed the induction of a stimulus- and cytokine-dependent innate immune memory phenotype that became apparent after the booster dose and was maintained eight months later in the absence of systemic inflammation.
Keywords: cytokine responses; inflammatory responses; innate immune memory; mRNA vaccine; proteomics.
Conflict of interest statement
L.A.B.J. is the scientific founder of Trained Therapeutix Discovery and Lemba Therapeutics. The remaining authors declare no conflicts of interest.
Figures

References
-
- Lauring A.S., Tenforde M.W., Chappell J.D., Gaglani M., Ginde A.A., Mcneal T., Ghamande S., Douin D.J., Talbot H.K., Casey J.D., et al. Clinical Severity of, and Effectiveness of MRNA Vaccines against, COVID-19 from Omicron, Delta, and Alpha SARS-CoV-2 Variants in the United States: Prospective Observational Study. BMJ. 2022;376:e069761. doi: 10.1136/bmj-2021-069761. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

