Advancing Vaccinology Capacity: Education and Efforts in Vaccine Development and Manufacturing across Africa
- PMID: 39066380
- PMCID: PMC11281707
- DOI: 10.3390/vaccines12070741
Advancing Vaccinology Capacity: Education and Efforts in Vaccine Development and Manufacturing across Africa
Abstract
Africa, home to the world's second-largest population of approximately 1.3 billion, grapples with significant challenges in meeting its medical needs, particularly in accessing quality healthcare services and products. The continent faces a continuous onslaught of emerging infectious diseases, exacerbating the strain on its already fragile public health infrastructure. The COVID-19 crisis highlighted the urgency to build local vaccine production capacity and strengthen the health infrastructure in general. The risks associated with a heavy reliance on imported vaccines were exposed during the COVID-19 pandemic, necessitating the need to nurture and strengthen the local manufacturing of vaccines and therapeutic biologics. Various initiatives addressing training, manufacturing, and regulatory affairs are underway, and these require increasing dedicated and purposeful financial investment. Building vaccine manufacturing capacity requires substantial investment in training and infrastructure. This manuscript examines the current state of education in vaccinology and related sciences in Africa. It also provides an overview of the continent's efforts to address educational needs in vaccine development and manufacturing. Additionally, it evaluates the initiatives aimed at strengthening vaccine education and literacy, highlighting successful approaches and ongoing challenges. By assessing the progress made and identifying the remaining obstacles, this review offers insights into how Africa can enhance its vaccine manufacturing capacity to respond to vaccine-preventable disease challenges.
Keywords: African countries; education; vaccine manufacturing; vaccinology.
Conflict of interest statement
No competing interests are disclosed by the authors.
Figures

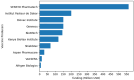
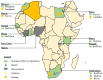
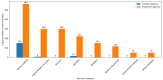
References
-
- Mihigo R., Anya B., Okeibunor J., Poy A., Machingaidze S., Wiysonge C.J. Routine immunization in the WHO African region: Progress, challenges and way forward. Afr. Health Monit. 2015;19:2–7.
-
- Wright C.Y., Kapwata T., Naidoo N., Asante K.P., Arku R.E., Cissé G., Simane B., Atuyambe L., Berhane K. Climate Change and Human Health in Africa in Relation to Opportunities to Strengthen Mitigating Potential and Adaptive Capacity: Strategies to Inform an African “Brains Trust”. Ann. Glob. Health. 2024;90:7. doi: 10.5334/aogh.4260. - DOI - PMC - PubMed
-
- Nyaruwata C. International Responses to Health Epidemics: An Analysis of Global Health Actors’ Responses to Persistent Cholera Outbreaks in Harare, Zimbabwe. BMJ Glob. Health. 2022;7:e009667.
Publication types
LinkOut - more resources
Full Text Sources

