Clinical and Diagnostic Features of Post-Acute COVID-19 Vaccination Syndrome (PACVS)
- PMID: 39066428
- PMCID: PMC11281408
- DOI: 10.3390/vaccines12070790
Clinical and Diagnostic Features of Post-Acute COVID-19 Vaccination Syndrome (PACVS)
Abstract
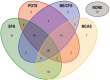
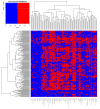
Post-acute COVID-19 vaccination syndrome (PACVS) is a chronic disease triggered by SARS-CoV-2 vaccination (estimated prevalence 0.02%). PACVS is discriminated from the normal post-vaccination state by altered receptor antibodies, most notably angiotensin II type 1 and alpha-2B adrenergic receptor antibodies. Here, we investigate the clinical phenotype using a study registry encompassing 191 PACVS-affected persons (159 females/32 males; median ages: 39/42 years). Unbiased clustering (modified Jaccard index) of reported symptoms revealed a prevalent cross-cohort symptomatology of malaise and chronic fatigue (>80% of cases). Overlapping clusters of (i) peripheral nerve dysfunction, dysesthesia, motor weakness, pain, and vasomotor dysfunction; (ii) cardiovascular impairment; and (iii) cognitive impairment, headache, and visual and acoustic dysfunctions were also frequently represented. Notable abnormalities of standard serum markers encompassing increased interleukins 6 and 8 (>80%), low free tri-iodine thyroxine (>80%), IgG subclass imbalances (>50%), impaired iron storage (>50%), and increased soluble neurofilament light chains (>30%) were not associated with specific symptoms. Based on these data, 131/191 participants fit myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and simultaneously also several other established dysautonomia syndromes. Furthermore, 31/191 participants fit none of these syndromes. In conclusion, PACVS could either be an outlier of ME/CFS or a dysautonomia syndrome sui generis.
Keywords: PACVS; cognitive impairment; interleukin-6; interleukin-8; malaise/chronic fatigue; peripheral nerve dysfunction; post-acute COVID-19 vaccination syndrome.
Conflict of interest statement
Harald Heidecke is co-owner and chief executive officer of CellTrend GmbH, Luckenwalde, Germany. The other authors have no conflicts of interest to declare.
Figures

References
-
- Semmler A., Mundorf A.K., Kuechler A.S., Schulze-Bosse K., Heidecke H., Schulze-Forster K., Schott M., Uhrberg M., Weinhold S., Lackner K.J., et al. Chronic Fatigue and Dysautonomia following COVID-19 Vaccination Is Distinguished from Normal Vaccination Response by Altered Blood Markers. Vaccines. 2023;11:1642. doi: 10.3390/vaccines11111642. - DOI - PMC - PubMed
-
- Carruthers B.M., van de Sande M.I., De Meirleir K.L., Klimas N.G., Broderick G., Mitchell T., Staines D., Powles A.C., Speight N., Vallings R., et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011;270:327–338. doi: 10.1111/j.1365-2796.2011.02428.x. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

