Equal Maintenance of Anti-SARS-CoV-2 Antibody Levels Induced by Heterologous and Homologous Regimens of the BNT162b2, ChAdOx1, CoronaVac and Ad26.COV2.S Vaccines: A Longitudinal Study Up to the 4th Dose of Booster
- PMID: 39066430
- PMCID: PMC11281708
- DOI: 10.3390/vaccines12070792
Equal Maintenance of Anti-SARS-CoV-2 Antibody Levels Induced by Heterologous and Homologous Regimens of the BNT162b2, ChAdOx1, CoronaVac and Ad26.COV2.S Vaccines: A Longitudinal Study Up to the 4th Dose of Booster
Abstract
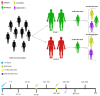
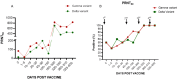
Several technological approaches have been used to develop vaccines against COVID-19, including those based on inactivated viruses, viral vectors, and mRNA. This study aimed to monitor the maintenance of anti-SARS-CoV-2 antibodies in individuals from Brazil according to the primary vaccination regimen, as follows: BNT162b2 (group 1; 22) and ChAdOx1 (group 2; 18). Everyone received BNT162b2 in the first booster while in the second booster CoronaVac, Ad26.COV2.S, or BNT162b2. Blood samples were collected from 2021 to 2023 to analyze specific RBD (ELISA) and neutralizing antibodies (PRNT50). We observed a progressive increase in anti-RBD and neutralizing antibodies in each subsequent dose, remaining at high titers until the end of follow-up. Group 1 had higher anti-RBD antibody titers than group 2 after beginning the primary regimen, with significant differences after the 2nd and 3rd doses. Group 2 showed a more expressive increase after the first booster with BNT162B2 (heterologous booster). Group 2 also presented high levels of neutralizing antibodies against the Gamma and Delta variants until five months after the second booster. In conclusion, the circulating levels of anti-RBD and neutralizing antibodies against the two variants of SARS-CoV-2 were durable even five months after the 4th dose, suggesting that periodic booster vaccinations (homologous or heterologous) induced long-lasting immunity.
Keywords: BNT162b2; COVID-19; ChAdOx1-S; CoronaVac; anti-RBD antibodies; dose booster; neutralizing antibodies; study longitudinal; vaccine effectiveness.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Queiroz M.A.F., Santiago A.M., de Brito W.B., Pereira K.A.S., de Brito W.B., da Silva Torres M.K., da Costa Lopes J., dos Santos E.F., da Costa F.P., de Sarges K.M.L., et al. Polymorphisms in the MBL2 Gene Are Associated with the Plasma Levels of MBL and the Cytokines IL-6 and TNF-α in Severe COVID-19. Front. Immunol. 2023;14:1151058. doi: 10.3389/fimmu.2023.1151058. - DOI - PMC - PubMed
-
- Rodrigues F.B.B., da Silva R., Santos E.F.d., de Brito M.T.F.M., da Silva A.L.S., de Meira Leite M., Póvoa da Costa F., de Nazaré do Socorro de Almeida Viana M., de Sarges K.M.L., Cantanhede M.H.D., et al. Association of Polymorphisms of IL-6 Pathway Genes (IL6, IL6R and IL6ST) with COVID-19 Severity in an Amazonian Population. Viruses. 2023;15:1197. doi: 10.3390/v15051197. - DOI - PMC - PubMed
-
- Malik Y.A. Properties of Coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020;42:3–11. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

