Systemic and immunotoxicity induced by topical application of perfluoroheptane sulfonic acid (PFHpS) or perfluorooctane sulfonic acid (PFOS) in a murine model
- PMID: 39066581
- PMCID: PMC11590111
- DOI: 10.1080/1547691X.2024.2371868
Systemic and immunotoxicity induced by topical application of perfluoroheptane sulfonic acid (PFHpS) or perfluorooctane sulfonic acid (PFOS) in a murine model
Abstract
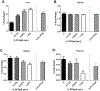
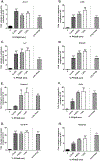
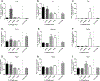
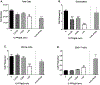
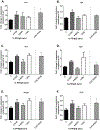
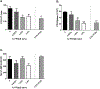
Per- and polyfluoroalkyl substances (PFAS) are a large group of synthetic surfactants of over 12,000 compounds that are incorporated into numerous products for their chemical and physical properties. Studies have associated PFAS with adverse health effects. Although there is a high potential for dermal exposure, these studies are lacking. The present study evaluated the systemic and immunotoxicity of subchronic 28- or 10-days of dermal exposure, respectively, to PFHpS (0.3125-2.5% or 7.82-62.5 mg/kg/dose) or PFOS (0.5% or 12.5 mg/kg/dose) in a murine model. Elevated levels of PFHpS were detected in the serum and urine, suggesting that absorption is occurring through the dermal route. PFHpS induced significantly increased relative liver weight, significantly decreased relative spleen and thymus weight, altered serum chemistries, and altered histopathology. Additionally, PFHpS significantly reduced the humoral immune response and altered immune subsets in the spleen, suggesting immunosuppression. Gene expression changes were observed in the liver, skin, and spleen of genes involved in fatty acid metabolism, necrosis, and inflammation. Immune-cell phenotyping identified significant decreases in B-cells and CD11b+ monocyte and/or macrophages in the spleen along with decreases in eosinophils and dendritic cells in the skin. These findings support PFHpS absorption through the skin leading to liver damage and immune suppression.
Keywords: PPAR; Perfluoroheptane sulfonic acid (PFHpS;), toxicity; dermal; immune; immunosuppression; liver damage.
Figures






References
-
- ATSDR (Agency for Toxic Substances and Disease Registry). 2021. Toxicological profile for perfluoroalkyls. Atlanta, GA. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=1117&tid=237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
