Housing mice near vs. below thermoneutrality affects drug-induced weight loss but does not improve prediction of efficacy in humans
- PMID: 39067024
- PMCID: PMC11380917
- DOI: 10.1016/j.celrep.2024.114501
Housing mice near vs. below thermoneutrality affects drug-induced weight loss but does not improve prediction of efficacy in humans
Abstract
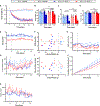
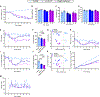
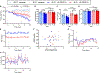
Evaluation of weight loss drugs is usually performed in diet-induced obese mice housed at ∼22°C. This is a cold stress that increases energy expenditure by ∼35% compared to thermoneutrality (∼30°C), which may overestimate drug-induced weight loss. We investigated five anti-obesity mechanisms that have been in clinical development, comparing weight loss in mice housed at 22°C vs. 30°C. Glucagon-like peptide-1 (GLP-1), human fibroblast growth factor 21 (hFGF21), and melanocortin-4 receptor (MC4R) agonist induced similar weight losses. Peptide YY elicited greater vehicle-subtracted weight loss at 30°C (7.2% vs. 1.4%), whereas growth differentiation factor 15 (GDF15) was more effective at 22°C (13% vs. 6%). Independent of ambient temperature, GLP-1 and hFGF21 prevented the reduction in metabolic rate caused by weight loss. There was no simple rule for a better prediction of human drug efficacy based on ambient temperature, but since humans live at thermoneutrality, drug testing using mice should include experiments near thermoneutrality.
Keywords: CP: Metabolism; FGF21; GDF15; GLP-1; MC4R; PYY; diet-induced obese mice; energy expenditure; glucose tolerance; housing temperature; human translatability; pharmaco-mediated weight loss; thermoneutrality.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.M.J., N.P., M.K.G., K.P., S.O., B.S.W., B.A., B.O.C., and R.E.K. are employed by Novo Nordisk A/S, and some are minor shareholders in Novo Nordisk A/S.
Figures



References
-
- Gordon CJ (1993). Temperature Regulation in Laboratory Rodents (Cambridge University Press; ). 10.1017/CBO9780511565595. - DOI
-
- John LM, Petersen N, Gerstenberg MK, Torz L, Pedersen K, Christoffersen B, and Kuhre RE (2022). Housing-temperature reveals energy intake counter-balances energy expenditure in normal-weight, but not diet-induced obese, male mice. Commun. Biol. 5, 946. 10.1038/s42003-022-03895-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

