Formaldehyde and the transient receptor potential ankyrin-1 contribute to electronic cigarette aerosol-induced endothelial dysfunction in mice
- PMID: 39067042
- PMCID: PMC11424888
- DOI: 10.1093/toxsci/kfae096
Formaldehyde and the transient receptor potential ankyrin-1 contribute to electronic cigarette aerosol-induced endothelial dysfunction in mice
Abstract
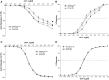
Electronic nicotine delivery systems (ENDS) aerosol exposures can induce endothelial dysfunction (ED) in healthy young humans and animals. Thermal degradation of ENDS solvents, propylene glycol, and vegetable glycerin (PG: VG), generates abundant formaldehyde (FA) and other carbonyls. Because FA can activate the transient receptor potential ankyrin-1 (TRPA1) sensor, we hypothesized that FA in ENDS aerosols provokes TRPA1-mediated changes that include ED and "respiratory braking"-biomarkers of harm. To test this, wild-type (WT) and TRPA1-null mice were exposed by inhalation to either filtered air, PG: VG-derived aerosol, or FA (5 ppm). Short-term exposures to PG: VG and FA-induced ED in female WT but not in female TRPA1-null mice. Moreover, acute exposures to PG: VG and FA stimulated respiratory braking in WT but not in TRPA1-null female mice. Urinary metabolites of FA (ie, N-1,3-thiazolidine-4-carboxylic acid, TCA; N-1,3-thiazolidine-4-carbonyl glycine, TCG) and monoamines were measured by LC-MS/MS. PG: VG and FA exposures significantly increased urinary excretion of both TCA and TCG in both WT and TRPA1-null mice. To confirm that inhaled FA directly contributed to urinary TCA, mice were exposed to isotopic 13C-FA gas (1 ppm, 6 h). 13C-FA exposure significantly increased the urine level of 13C-TCA in the early collection (0 to 3 h) supporting a direct relationship between inhaled FA and TCA. Collectively, these data suggest that ENDS use may increase CVD risk dependent on FA, TRPA1, and catecholamines, yet independently of either nicotine or flavorants. This study supports that levels of FA in ENDS-derived aerosols should be lowered to mitigate CVD risk in people who use ENDS.
Keywords: aldehydes; cardiovascular disease; electronic nicotine delivery systems (ENDS); endothelium; irritants; tobacco.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
All authors declare no conflicts of interest in this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Food and Drug Administration/Center for Tobacco Products, or the American Heart Association.
Figures





References
-
- Anand U, Otto WR, Facer P, Zebda N, Selmer I, Gunthorpe MJ, Chessell IP, Sinisi M, Birch R, Anand P. 2008. TRPA1 receptor localisation in the human peripheral nervous system and functional studies in cultured human and rat sensory neurons. Neurosci Lett. 438(2):221–227. 10.1016/j.neulet.2008.04.007. - DOI - PubMed
-
- Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, et al. 2008. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. JClinInvest. 118(7):2574–2582. 10.1172/JCI34886. - DOI - PMC - PubMed
-
- Arastoo S, Haptonstall KP, Choroomi Y, Moheimani R, Nguyen K, Tran E, Gornbein J, Middlekauff HR. 2020. Acute and chronic sympathomimetic effects of e-cigarette and tobacco cigarette smoking: role of nicotine and non-nicotine constituents. Am J Physiol Heart Circ Physiol. 319(2):H262–H270. 10.1152/ajpheart.00192.2020. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

