Copper-mediated neurotoxicity and genetic vulnerability in the background of neurodegenerative diseases in C. elegans
- PMID: 39067045
- PMCID: PMC11424883
- DOI: 10.1093/toxsci/kfae092
Copper-mediated neurotoxicity and genetic vulnerability in the background of neurodegenerative diseases in C. elegans
Abstract
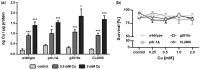
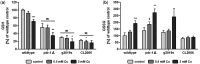
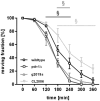
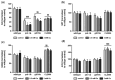
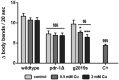
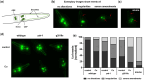
The mechanisms associated with neurodegenerative diseases, such as Alzheimer's disease (AD) and Parkinson's disease (PD), have yet to be fully characterized, and genetic as well as environmental factors in their disease etiology are underappreciated. Although mutations in genes such as PARKIN and LRRK2 have been linked to PD, the idiopathic component of the disease suggests a contribution of environmental risk factors, including metals, such as copper (Cu). Cu overexposure has been reported to cause oxidative stress and neurotoxicity, but its role in neurodegenerative diseases is rarely studied. Using Caenorhabditis elegans (C. elegans) as a model organism for neurotoxicity, we assessed the effects of Cu oversupply in AD and PD models. Our findings reveal that although copper treatment did not induce neurodegeneration in wild-type worms or the AD model, it significantly exacerbated neurodegeneration in the PD-associated mutants PARKIN and LRRK2. These results suggest that genetic predisposition for PD enhances the sensitivity to copper toxicity, highlighting the multifactorial nature of neurodegenerative diseases. Furthermore, our study provides insight into the mechanisms underlying Cu-induced neurotoxicity in PD models, including disruptions in dopamine levels, altered dopamine-dependent behavior and degraded dopaminergic neurons. Overall, our novel findings contribute to a better understanding of the complex interactions between genetic susceptibility, environmental factors, and neurodegenerative disease pathogenesis, emphasizing the importance of a tightly regulated Cu homeostasis in the etiology of PD. Copper oversupply exacerbated neurodegeneration in Caenorhabditis elegans models of Parkinson's disease, highlighting the genetic susceptibility and emphasizing the crucial role of tightly regulated copper homeostasis in Parkinson's disease pathogenesis.
Keywords: C. elegans; copper toxicity; genetic predisposition; neurodegenerative diseases.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

