A novel drug prejudice scaffold-imidazopyridine-conjugate can promote cell death in a colorectal cancer model by binding to β-catenin and suppressing the Wnt signaling pathway
- PMID: 39067696
- PMCID: PMC12147644
- DOI: 10.1016/j.jare.2024.07.022
A novel drug prejudice scaffold-imidazopyridine-conjugate can promote cell death in a colorectal cancer model by binding to β-catenin and suppressing the Wnt signaling pathway
Abstract
Introduction: Globally, colorectal cancer (CRC) is the third most common type of cancer, and its treatment frequently includes the utilization of drugs based on antibodies and small molecules. The development of CRC has been linked to various signaling pathways, with the Wnt/β-catenin pathway identified as a key target for intervention.
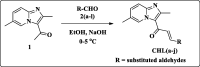
Objectives: We have explored the impact of imidazopyridine-tethered chalcone-C (CHL-C) in CRC models.
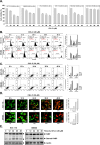
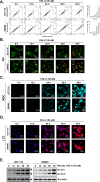
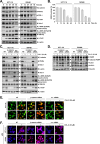
Methods: To determine the influence of CHL-C on apoptosis and autophagy, Western blot analysis, annexin V assay, cell cycle analysis, acridine orange staining, and immunocytochemistry were performed. Next, the activation of the Wnt/β-catenin signaling pathway and the anti-cancer effects of CHL-C in vivo were examined in an orthotopic HCT-116 mouse model.
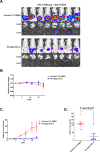
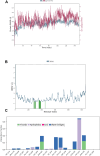
Results: We describe the synthesis and biological assessment of the CHL series as inhibitors of the viability of HCT-116, SW480, HT-29, HCT-15, and SNU-C2A CRC cell lines. Further biological evaluations showed that CHL-C induced apoptosis and autophagy in down-regulated β-catenin, Wnt3a, FZD-1, Axin-1, and p-GSK-3β (Ser9), and up-regulated p-GSK3β (Tyr216) and β-TrCP. In-depth analysis using structure-based bioinformatics showed that CHL-C strongly binds to β-catenin, with a binding affinity comparable to that of ICG-001, a well-known β-catenin inhibitor. Additionally, our in vivo research showed that CHL-C markedly inhibited tumor growth and triggered the activation of both apoptosis and autophagy in tumor tissues.
Conclusion: CHL-C is capable of inducing apoptosis and autophagy by influencing the Wnt/β-catenin signaling pathway.
Keywords: Apoptosis; Autophagy; CHL-C; Colorectal cancer; Wnt/ β −catenin.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Siegel R.L., Wagle N.S., Cercek A., Smith R.A., Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233–254. - PubMed
-
- Hani U., Honnavalli Y.K., Begum M.Y., Yasmin S., Osmani R.A.M., Ansari M.Y. Colorectal cancer: A comprehensive review based on the novel drug delivery systems approach and its management. J Drug Deliv Sci Tec. 2021:63.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

