Density of tertiary lymphoid structures predicts clinical outcome in breast cancer brain metastasis
- PMID: 39067874
- PMCID: PMC11284839
- DOI: 10.1136/jitc-2024-009232
Density of tertiary lymphoid structures predicts clinical outcome in breast cancer brain metastasis
Abstract
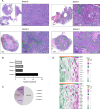
Background: Patients with breast cancer brain metastases (BCBM) experience a rapid decline in their quality of life. Recently, tertiary lymphoid structures (TLSs), analogs of secondary lymphoid organs, have attracted extensive attention. However, the potential clinical implications of TLSs in BCBMs are poorly understood. In this study, we evaluated the density and composition of TLSs in BCBMs and described their prognostic value.
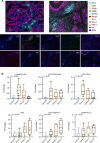
Methods: Clinicopathological data were collected from 98 patients (2015-2021). TLSs were evaluated, and a TLS scoring system was constructed. Differences in progression-free survival (PFS) and overall survival (OS) between groups were calculated using the Kaplan-Meier method. Immunohistochemistry and multiplex immunofluorescence (mIF) were used to assess TLSs heterogeneity.
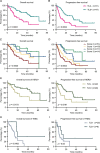
Results: TLSs were identified in 47 patients with BCBM. High TLSs density indicated favorable survival (OS, p=0.003; PFS, p<0.001). TLS was positively associated with OS (p=0.0172) and PFS (p=0.0161) in the human epidermal growth factor receptor type 2-positive subtype, and with prolonged OS (p=0.0482) in the triple-negative breast cancer subtype. The mIF results showed significant differences in the percentages of T follicular helper (Tfh) cells, M2 macrophages, cytotoxic T lymphocytes, and CD8+TIM-3+ T lymphocytes between the groups of TLS scores 0-3 (cytotoxic T lymphocytes, p=0.044; Tfh, p=0.021; M2 macrophages, p=0.033; CD8+TIM-3+ T lymphocytes, p=0.018). Furthermore, novel nomograms incorporating the TLS scores and other clinicopathological predictors demonstrated prominent predictability of the 1-year, 3-year, and 5-year outcomes of BCBMs (area under the curve >0.800).
Conclusion: Our results highlight the impact of TLSs abundance on the OS and PFS of patients with BCBM. Additionally, we described the immune composition of TLSs and proposed novel nomograms to predict the prognosis of patients with BCBM.
Keywords: Central Nervous System Cancer; Relapse; Survivorship; Tumor Microenvironment.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures


Similar articles
-
Localization and density of tertiary lymphoid structures associate with molecular subtype and clinical outcome in colorectal cancer liver metastases.J Immunother Cancer. 2023 Feb;11(2):e006425. doi: 10.1136/jitc-2022-006425. J Immunother Cancer. 2023. PMID: 36759015 Free PMC article.
-
The Presence of Tertiary Lymphoid Structures Provides New Insight Into the Clinicopathological Features and Prognosis of Patients With Breast Cancer.Front Immunol. 2022 May 19;13:868155. doi: 10.3389/fimmu.2022.868155. eCollection 2022. Front Immunol. 2022. PMID: 35664009 Free PMC article.
-
Distribution and density of tertiary lymphoid structures predict clinical outcome in intrahepatic cholangiocarcinoma.J Hepatol. 2022 Mar;76(3):608-618. doi: 10.1016/j.jhep.2021.10.030. Epub 2021 Nov 15. J Hepatol. 2022. PMID: 34793865
-
Immune cells within tertiary lymphoid structures are associated with progression-free survival in patients with locoregional recurrent breast cancer.Cancer Med. 2024 Jan;13(1):e6864. doi: 10.1002/cam4.6864. Epub 2023 Dec 22. Cancer Med. 2024. PMID: 38133211 Free PMC article.
-
Tertiary lymphoid structures in the era of cancer immunotherapy.Nat Rev Cancer. 2019 Jun;19(6):307-325. doi: 10.1038/s41568-019-0144-6. Nat Rev Cancer. 2019. PMID: 31092904 Review.
Cited by
-
Imaging antigen processing and presentation in cancer.Immunother Adv. 2025 Feb 4;5(1):ltaf002. doi: 10.1093/immadv/ltaf002. eCollection 2025. Immunother Adv. 2025. PMID: 40265075 Free PMC article. Review.
-
B cells and tertiary lymphoid structures in tumors: immunity cycle, clinical impact, and therapeutic applications.Theranostics. 2025 Jan 1;15(2):605-631. doi: 10.7150/thno.105423. eCollection 2025. Theranostics. 2025. PMID: 39744696 Free PMC article. Review.
-
Identification and characterization of tertiary lymphoid structures in brain metastases.Acta Neuropathol Commun. 2025 May 3;13(1):91. doi: 10.1186/s40478-025-02007-x. Acta Neuropathol Commun. 2025. PMID: 40319321 Free PMC article.
-
Density of tertiary lymphoid structures predict clinical outcome in hepatoblastoma.Pediatr Res. 2025 Jul 9. doi: 10.1038/s41390-025-04210-x. Online ahead of print. Pediatr Res. 2025. PMID: 40634689
-
Intratumoural CD8+ CXCR5+ follicular cytotoxic T cells have prognostic value and are associated with CD19+ CD38+ B cells and tertiary lymphoid structures in colorectal cancer.Cancer Immunol Immunother. 2024 Dec 30;74(1):36. doi: 10.1007/s00262-024-03887-z. Cancer Immunol Immunother. 2024. PMID: 39739032 Free PMC article.
References
-
- Onkar SS, Carleton NM, Lucas PC, et al. . The great immune escape: understanding the divergent immune response in breast cancer subtypes. Cancer Discov 2023;13:23–40. 10.1158/2159-8290.CD-22-0475 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
