Targeting myeloid-derived suppressor cells promotes antiparasitic T-cell immunity and enhances the efficacy of PD-1 blockade (15 words)
- PMID: 39068159
- PMCID: PMC11283557
- DOI: 10.1038/s41467-024-50754-7
Targeting myeloid-derived suppressor cells promotes antiparasitic T-cell immunity and enhances the efficacy of PD-1 blockade (15 words)
Erratum in
-
Author Correction: Targeting myeloid-derived suppressor cells promotes antiparasitic T-cell immunity and enhances the efficacy of PD-1 blockade.Nat Commun. 2024 Aug 27;15(1):7374. doi: 10.1038/s41467-024-51832-6. Nat Commun. 2024. PMID: 39191752 Free PMC article. No abstract available.
Abstract
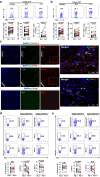
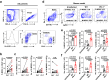
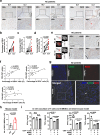
Immune exhaustion corresponds to a loss of effector function of T cells that associates with cancer or chronic infection. Here, our objective was to decipher the mechanisms involved in the immune suppression of myeloid-derived suppressor cells (MDSCs) and to explore the potential to target these cells for immunotherapy to enhance checkpoint blockade efficacy in a chronic parasite infection. We demonstrated that programmed cell-death-1 (PD-1) expression was significantly upregulated and associated with T-cell dysfunction in advanced alveolar echinococcosis (AE) patients and in Echinococcus multilocularis-infected mice. PD-1 blockade ex vivo failed to reverse AE patients' peripheral blood T-cell dysfunction. PD-1/PD-L1 blockade or PD-1 deficiency had no significant effects on metacestode in mouse model. This was due to the inhibitory capacities of immunosuppressive granulocytic MDSCs (G-MDSCs), especially in the liver surrounding the parasite pseudotumor. MDSCs suppressed T-cell function in vitro in an indoleamine 2, 3 dioxygenase 1 (IDO1)-dependent manner. Although depleting MDSCs alone restored T-cell effector functions and led to some limitation of disease progression in E. multilocularis-infected mice, combination with PD-1 blockade was better to induce antiparasitic efficacy. Our findings provide preclinical evidence in support of targeting MDSC or combining such an approach with checkpoint blockade in patients with advanced AE. (200 words).
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

